
On Wednesday, the Royal Swedish Academy of Sciences awarded the Nobel Prize in Chemistry to a pair of female scientists: Jennifer Doudna, a biochemist at the University of California, Berkeley and Emmanuelle Charpentier, microbiologist at the Max Planck Institute for Infection Biology in Berlin, Germany. The team became the first pair of women to share a Nobel Prize for a scientific discovery, and just the fifth and sixth women to win the Nobel in Chemistry in the prize’s history.
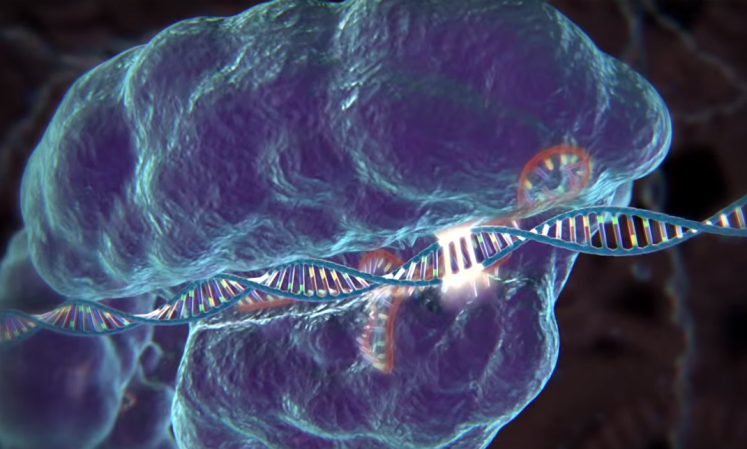
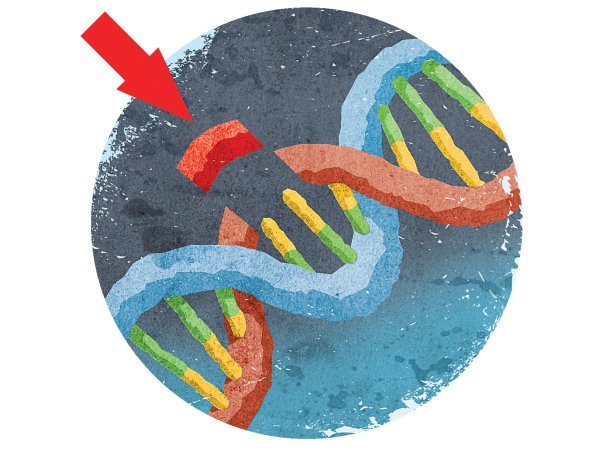
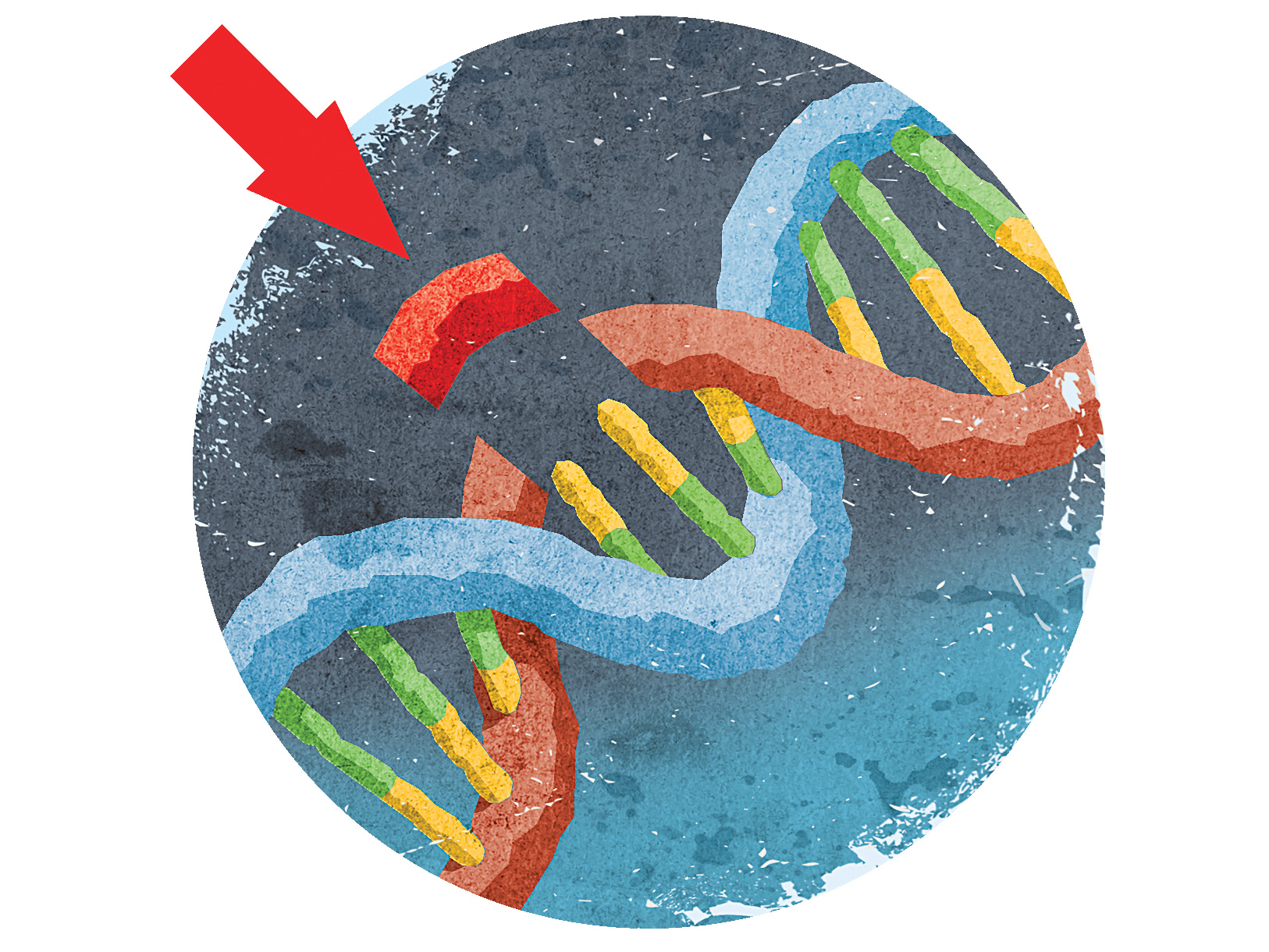
While Nobel prizes are often granted for work done decades ago—that’s typically how long it takes for a finding to prove to be truly groundbreaking for the field—Doudna and Charpentier made these contributions just a few years ago. Both women discovered a mechanism through which a bacterial enzyme, dubbed Cas9, can slice DNA, and how an accompanying set of RNA molecules called CRISPR can instruct the enzyme on where to cut. As such, the CRISPR-Cas9 duo could cut, remove, and add DNA into a genome in whichever spot it’s needed, all with relative ease and accuracy.
The applications for this technology are vast. Since its discovery, scientists around the world have employed the CRISPR-Cas9 system in a number of different ways: Plant biologists can tweak the genomes of fruits and vegetables to produce higher quality, more desirable produce and grains, and ones that can potentially withstand certain diseases or changes in climate. Microbiologists can tweak bacteria to create therapeutic drugs. However, the most promising and significant use of the technology is its potential for altering human cells.
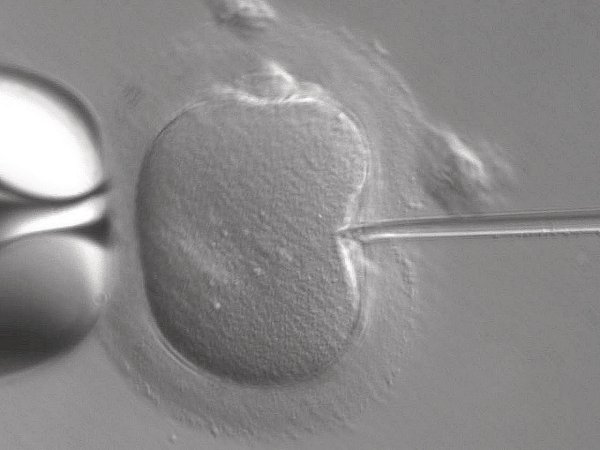
The CRISPR-Cas9 system allows researchers to edit any portion of the human genome they desire, and that capability has been fraught with ethical controversy. Tweaking so-called germline cells, which include the DNA inside sperm and eggs, means that any change to the genome would show up in every subsequent generation. Slicing somatic cells, however, which include all cells aside from sperm and egg cells, would only affect the individual being treated.
Since CRISPR-Cas9 was discovered, its ability to edit germline cells has given scientists, including Jennifer Doudna, pause. Over the years, scientists worldwide have attempted to enforce moratoriums on editing viable human embryos using CRISPR-Cas9 for any purposes other than therapeutic, such as treating an incurable genetic condition. However, those lines have been crossed. In 2018, He Jiankui, a Chinese scientist, edited the genomes of viable human embryos (leading to the birth of supposedly healthy twin girls). His aim was to make them impervious to HIV infection, but the genes involved also affect other aspects of health—and there’s no telling which other genes might have inadvertently been edited in the process. Scientists around the world, including in China, have condemned this work, and as recently as September of 2020, an international committee of experts said that CRISPR is not ready for use in viable human embryos.
CRISPR-Cas9 isn’t the first DNA editing technology out there. Two other technologies—zinc finger nuclease and TALENS—work similarly to CRISPR-Cas9, but do so with far less ease and precision. It’s CRISPR-Cas9′s efficiency that makes it so promising. Since 2012, a number of genetic companies have been formed whose sole focus is using the technology to cure genetic diseases. For example, CRISPR Therapeutics has ongoing clinical trials employing CRISPR-Cas9 to fix sickle cell anemia, as well as another blood condition known as beta thalassemia. Other companies are working on designing therapeutics for type 1 diabetes, cystic fibrosis, and hemophilia, among others.