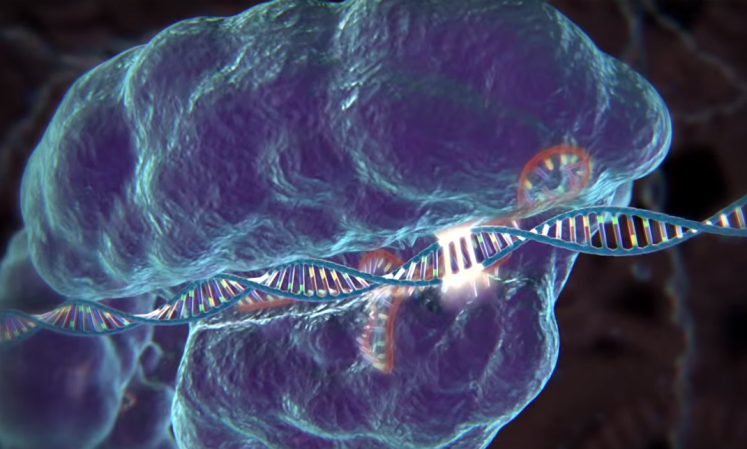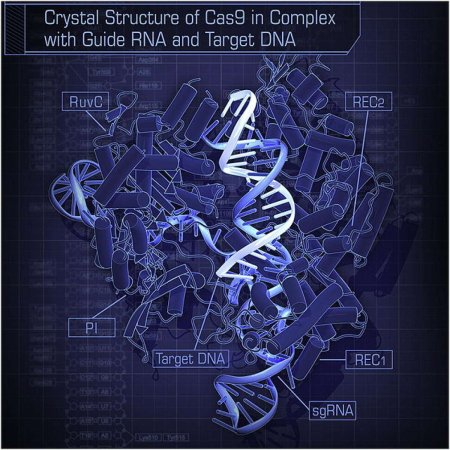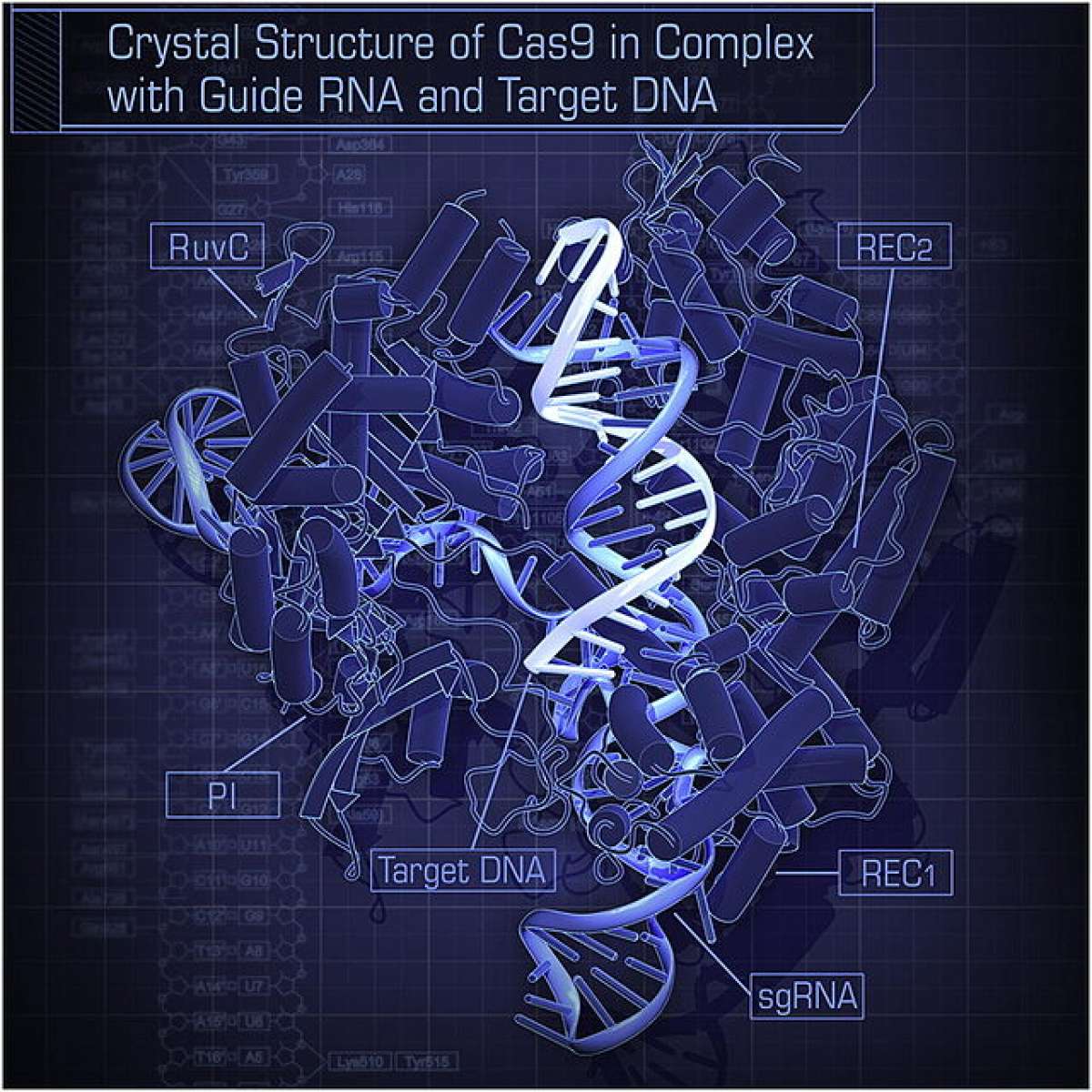


This week saw a resolution to the grueling legal battle between the University of California at Berkeley and the Broad Institute over the discovery of the novel, cheap, and easy-to-use gene-editing technique known as CRISPR. The technique’s original discoverers, Berkeley’s Jennifer Doudna and Emmanuelle Charpentier, lost a patent battle to the Broad Institute’s Feng Zhang over who first discovered that the technique could be used to edit human and animal DNA—a feat that has massive potential to treat and cure diseases.
But what still hangs in the air, detailed in a report out today in the journal Science, is who will be allowed to use this technology and in what way. Despite the fact that the patent rights to CRISPR were being disputed in court, private companies have seized on the opportunity to use this technology to research disease treatments, and entering into licensing agreements with the research institutions to gain exclusive rights to use CRISPR in a particular way to treat a particular disease. That would mean that no other company could do similar research. How can this be?
A set of guidelines from the National Institutes of Health in 1999 and another, called the “Nine Points”, that was agreed upon by a number of universities (including UC Berkeley, Harvard, and MIT) in 2007 state that any research tools that a research university invents should be made publicly available to any scientists or institution that wants to use it.
But most discoveries aren’t actually research tools, says Jorge Contreras, an intellectual property lawyer at the University of Utah and a co-author of today’s report. So, for example if someone at a research university discovers a new molecule that’s effective against a particular protein that would lead to a new way to treat a disease, that’s a therapy and it’s appropriate to license it exclusively.
Drawing the line between a research tool and a therapy is incredibly important. Take PCR, for example. The polymerase chain reaction, discovered in 1985, allows researchers to select a tiny and specific sample of DNA, and replicate it billions of times. Today, PCR helps scientists study bacteria, viruses, and genetic diseases—and it’s helped to discover many vital treatments and breakthroughs. Nearly every lab in the world now uses PCR, for any reason they want. That’s because it’s considered a research tool.
So what’s CRISPR? That’s the main question that needs to be answered, says Contreras. Currently, it’s not considered a research tool. Because of that, private companies have already entered into what’s called surrogate licensing agreements with both Broad (which is a collaboration between MIT and Harvard) and UC Berkeley. “That’s where we think CRISPR crosses the line,” Contreras says.
Contreras is afraid of what would happen if CRISPR remains where it is. For example, the private company Juno therapeutics has entered into surrogate licensing agreements with MIT and the Broad Institute to do research using CRISPR and the immunotherapy cancer treatment called CAR T-cell treatment. The treatment, which is still in clinical trials, is thought by many to revolutionize the way cancer is treated, and perhaps CRISPR could significantly help that. But Contreras’ concern is what happens if Juno folds, or is subject to lawsuits? The rights that it (or any other company) has could be tied up in courts for decades, delaying vital research. If Juno went bankrupt, no one else could use CRISPR and CAR T together to develop effective therapies. “This strikes me as a real shame and a loss to society,” says Contreras. Further, if other companies did apply to that company to use their licensed CRISPR therapy, that company can decide not to give it to them, especially if they are a competitor or if they think they will do the research that that company wants to do now in the future.
But Contreras says that because CRISPR is very much still in the research phase (and parties are still fighting over who owns the patents on these discoveries), it’s an ideal time to rethink how CRISPR should be viewed. “There can be some renegotiation of the basic CRISPR licensing,” he says.
Contreras says the recent court decision will almost surely be appealed. And he doesn’t think anything major will happen until those court decisions have been finalized. But in the meantime, private companies who want to be in this market are already positioning themselves and are paying a lot of money for some of these licenses that they think they are going to need.
To ensure that everyone who wants to do research on CRISPR has access, Contreras says the NIH should go back and rethink its old 1999 policy to ensure there’s no gray area that CRISPR can slip through, and that all broad research platforms, including something like CRISPR, should be licensed non-exclusively. “CRISPR definitely crossed over the line, and it should go back behind the line.”