
Click here to see all of PopSci’s COVID-19 coverage.
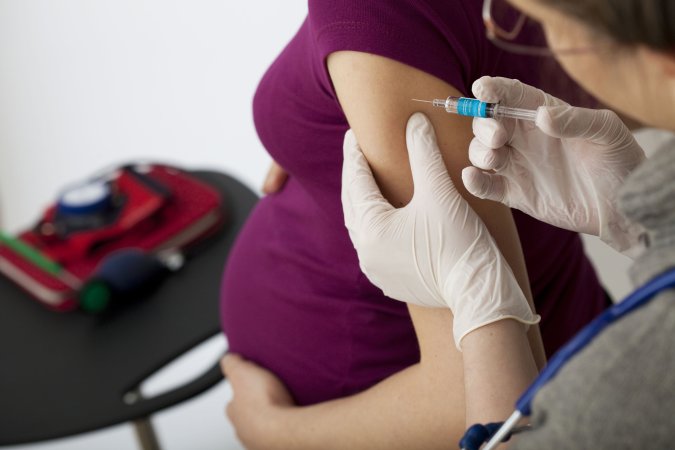
It will be impossible to bring the COVID-19 pandemic to its heels until life saving vaccines are available globally. That is a fact the world is increasingly having to reckon with.
But for now vaccinating a majority of the world’s population remains far from reach as experts argue over how, exactly, to scale up vaccine production and distribute doses equitably around the world.
Last week, the United States made waves by announcing it would back a waiver of intellectual property protections on COVID-19 vaccine technology—a move proponents say could remove legal barriers and make it easier for more countries, especially lower income ones, to make and dole out doses. “This is a global health crisis, and the extraordinary circumstances of the COVID-19 pandemic call for extraordinary measures,” said US Trade Representative Katherine Tai in a statement.
Advocates say the move signals a US commitment to addressing vaccine inequity and demonstrates a willingness to say to the pharmaceutical industry—which has received billions of dollars in public money, including for vaccine development and through purchase agreements—that public health outweighs profit.
“I think it’s certainly symbolic—symbolic in a good way,” says Georgetown University global health law professor Lawrence Gostin. “After all the destruction we did on the global stage under the last administration, this is a huge reputational advance for the US”
But others—including big pharmaceutical companies—say it’s a distraction from the real obstacles at hand: a worldwide shortage of raw materials, trained personnel, and other infrastructure necessary to make lifesaving COVID-19 shots. “There’s a mismatch. We’re looking at the law for answers but this is not a legal problem,” says Ana Santos Rutschman, an assistant professor of health law at Saint Louis University School of Law. “Tinkering with IP,” she says, is “not going to magically increase production overnight.”
Since the debut of COVID-19 vaccines late last year, wealthier countries have monopolized the limited production system by placing large advance orders—effectively crowding out middle- and low-income countries.
[Read more: We vetted popular at-home COVID-19 tests. Here’s what we learned.]
About half of the 1.3 billion vaccine doses delivered globally so far have been administered in the United States and China alone. The United States has provided at least one shot to more than 45 percent of the country’s population, according to a University of Oxford database. Meanwhile, very few doses have been administered in other parts of the world. In Brazil and India—two countries facing huge COVID-19 surges and staggering death tolls—only 15 percent and 10 percent of their populations, respectively, have been at least partially vaccinated. That figure for the continent of Africa is 1 percent, according to the Oxford database.
“The gap is very tragic between the have and have-nots,” World Health Organization director general Tedros Adhanom Ghebreyesus said on Saturday. Covax, a global initiative for vaccine distribution, has shipped less than a quarter of its target so far, The New York Times reported. And researchers have forecasted it could take years to reach levels close to herd immunity in the lowest-income countries. “We have the technology to save the world, and we’re not sharing it. We’re hoarding it,” Gostin says. “It’s absolutely unforgivable, morally.”
The virus will continue to spread and surge wherever it’s left unchecked, experts say, giving it ample opportunity to mutate into potentially more contagious, deadly, or vaccine-resistant versions. “We really need to protect other countries to keep ourselves safe,” says Graham Dutfield, a professor of international governance at the University of Leeds. “We won’t be assured to be safe until everybody’s safe.”
In response, a coalition of about 100 countries and international groups has petitioned World Trade Organization members for months to agree to temporarily waive IP protections on COVID-19 vaccines. Without broad intervention, they argue, holders of vaccine patents could refuse to license their technology to other companies. Under the proposal (which could take weeks or months to negotiate and finalize) independent manufacturers would be allowed to produce and distribute what would be otherwise patent-protected.
If enacted, the IP waiver would be historic. Previous public health crises have led to exceptional legal strategies aimed at quickly ramping up the production and mass adoption of groundbreaking medicines, drugs, and inventions.
During World War II, the US government compelled industry and academia to collaborate in order to scale up the production of penicillin for soldiers, Dutfield says. “They essentially forced industry and universities to basically pool all their knowledge,” he says. “It was hugely effective. The US went from producing nothing to producing vast amounts in about six months.”
A few decades later, Swedish carmaker Volvo decided to open its patent for a novel, three-point seatbelt open to competitors in order to widely disseminate the lifesaving design. And in the early 21st century, many countries were able to obtain licenses on patented drugs for the treatment of HIV/AIDS in order to quickly address the global epidemic.
But there’s a clear distinction between that crisis and the one we are still currently in, says Michael Merson, a research professor at NYU School of Global Public Health and professor of global health at Duke University. “There was the immediate capability to make these drugs—that’s not the case with these vaccines,” he says. “It’s not the same. I would love it if it were.”
[Read more: What the first year of COVID tells us about the next]
Critics of the IP waiver say it wouldn’t address any of these practical problems at the root of COVID-19 vaccine scarcity and inequity. Even without the obstacle of patents, it could take a year or more to set up new factories, train skilled personnel, obtain raw materials and set up distribution hubs. If the waiver is analogous to openly sharing the vaccines’ recipe, we still need to help new chefs source the ingredients and master the cooking techniques, says Prashant Yadav, a supply chain expert and senior fellow at the Center for Global Development. “First we solve the patent issue,” he says “then we need to solve everything else.”
Vaccines like the ones produced by Moderna and Pfizer use messenger RNA technology, which requires skilled scientists and technicians. New vaccine manufacturing sites will also need administrators and regulatory oversight to ensure the quality and safety of their products, as well as access to scarce materials including vials and stoppers. “All of that is in short supply,” Yadav says, and will take months to obtain. “We are talking about the earliest, earliest—if someone had everything in place—of 12 months.”
Yadav and others have floated another legal option they think could shave months or years off of the ramping-up process if big pharmaceutical companies agree to it: voluntary licensing. Under a voluntary licensing framework—which has been used before with treatments for HIV/AIDS and drug-resistant tuberculosis—patent-holders would directly work with new sites, coaching them through the manufacturing process as they scale up. The drug companies would also be able to include quality standards in their agreement. Rather than handing over the cookbook, Yadav says, “You’re sending the chef along with the recipe.”
In the meantime, some like Merson are urging existing, established vaccine makers to pump up their output and get out as many doses as quickly as possible. “We need to do everything we can to get vaccine supply up and out,” he says. “There needs to be more urgency. We’ve got to deal with this crisis now.”