
When the first round of COVID vaccines were authorized in December of 2020, they were developed in record time, due in large part to a flood of public funding that sped up the clinical trial process. They also far exceeded expectations for preventing disease and death—the White House estimates that two million lives have been saved by vaccines so far. But as SARS-CoV-2 has adapted to a population with widespread immunity, the vaccines have proven poorly equipped to stop transmission of variants like BA.5, and their efficacy has waned among the elderly, requiring repeated boosters.
At a White House summit on July 26, called “The Future of COVID-19 vaccines,” the Biden administration brought together pharmaceutical leaders, disease researchers, and federal public health officials to figure out next steps. The aim of the summit, top White House advisors said, was to chart a roadmap to vaccines that could resist variants and halt, rather than blunt, coronavirus transmission.
“That’s the holy grail,” said Anthony Fauci, director of the National Institute of Allergy and Infectious Disease, and the White House Chief Medical Advisor. “Durability, breadth, acquisition, transmission.”
However, the summit didn’t come with concrete proposals on how to accelerate the development of those tools. “In order to make that a reality, there’s a tremendous amount of work,” said Ashish Jha, the White House COVID-19 Response Coordinator. “This administration is fully committed to doing that work.”
“I hope that they invest the resources that are needed for Operation Warp Speed 2.0,” says Michal Tal, an immunologist at Stanford University who has studied variation in COVID-vaccine responses. “We’ve needed to launch that as soon as Operation Warp Speed 1.0 was rolling.”
A vaccine for many viruses
The US vaccine strategy at present involves requesting that vaccine manufacturers like Pfizer and Moderna tweak their mRNA formulas to more closely resemble the mutated spike proteins of the BA.4/BA.5 variant. But because those updated shots need to go through the clinical approval process, they likely won’t be ready until this fall.
However, because the latest Omicron variants emerged just a few months apart, it’s very possible that BA.4 and BA.5 will no longer be dominant by then. “I feel like we’re chasing a ghost,” Tal says.
One strategy discussed at the summit involves developing a pan-coronavirus vaccine. The idea is that a vaccine could spark an immune response that would target a common feature of all SARS-CoV-2 variants, or even all SARS-like viruses. One approach to that, outlined by Sandeep Reddy, the chief medical officer of the pharmaceutical company ImmunityBio, which is entering Phase 3 trials for a vaccine in South Africa, is to train immune cells to recognize not just the spikes of a coronavirus, but the internal machinery that lets the virus reproduce.
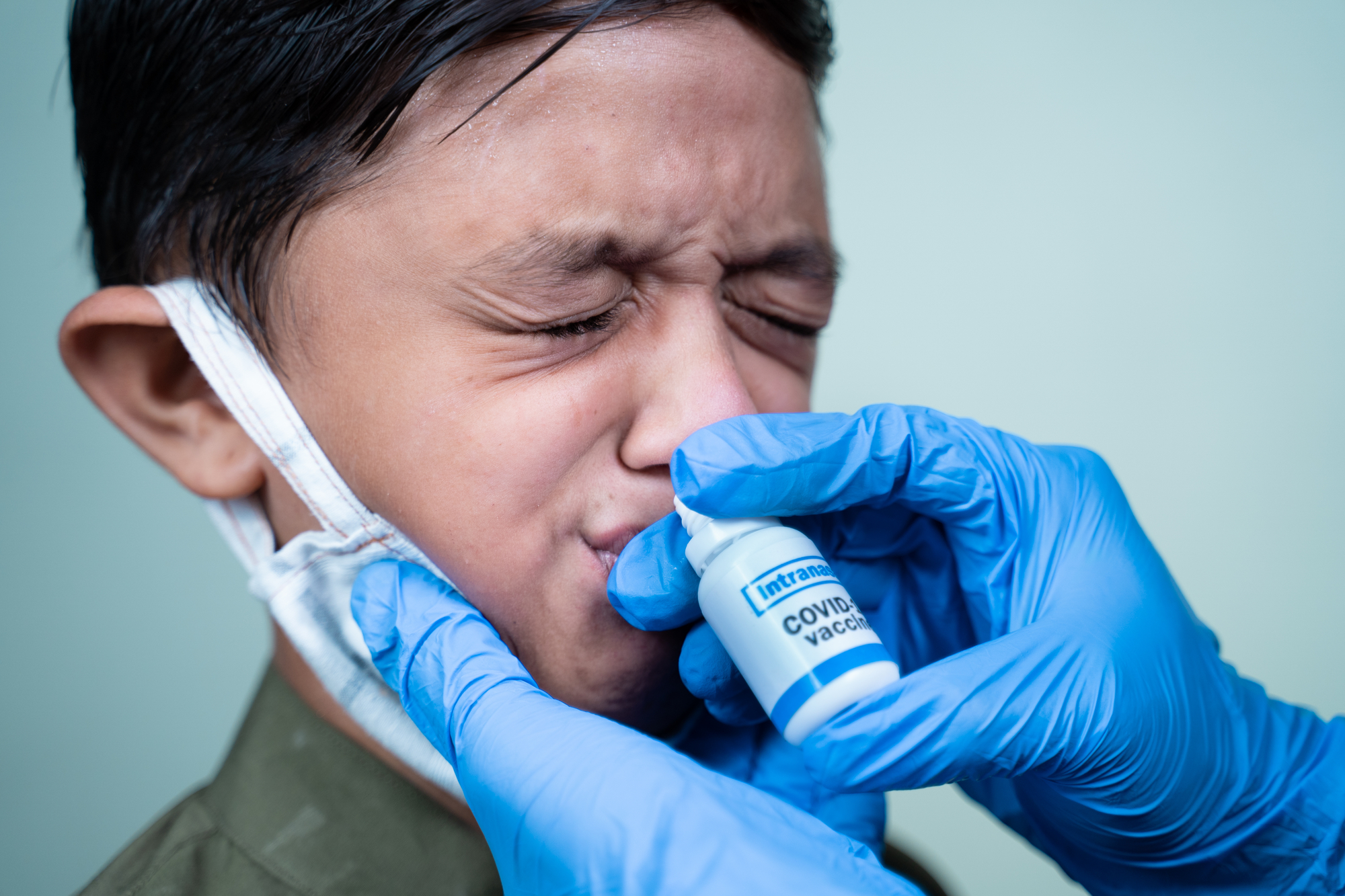
From shots to sprays and pills
So far, existing vaccines have been durable at preventing severe illness and death in the face of new variants. That means a pan-coronavirus vaccine might be useful against a radically different variant or a novel virus, but it won’t necessarily stop the spread of existing variants.
To do that, immunologists have increasingly pointed towards the need for vaccines that provoke an immune response in the mucosal linings of the throat, mouth, and nose. “This is where the actual battle rages day to day,” Tal says.
While injected vaccines have been very successful at triggering antibodies in the blood and protecting vital organs, mucosal tissues are defended by an entirely different type of antibody. Research co-authored by Tal shows that injected COVID vaccines did produce fleeting mucosal antibodies in some people, and the presence of those antibodies made a person less likely to experience a breakthrough infection. The results suggest that a vaccine specifically designed to hit mucosal tissue might be particularly effective at stopping SARS-CoV-2 transmission.
Pharmaceutical companies are pursuing that goal in a number of ways, the most common being a nasal spray vaccine. Akiko Iwasaki, a professor of immunology at Yale University and a panelist at the summit, founded a firm that’s specifically investigating whether mRNA boosters can be given through the nose. A team led by NIAID scientists also investigated delivering the AstraZeneca vaccine nasally, which reduced viral shedding in hamsters and monkeys. But another biotech group, the US-based Vaxart, is running clinical trials on a vaccine pill aimed at activating mucosal immunity in the lining of the gut.
A number of immunologists pointed to success with the AstraZeneca nasal FluMist vaccine. In the early 2010s, federal regulators actually recommended it over flu shots for children, Marty Moore, chief scientific officer of Meissa Vaccines, pointed out during a panel. “It was more durable, it could reduce transmission—these are the principles of mucosal immunization that we’re aiming for.” It did decline in popularity and use after manufacturing problems, but Tal says that had to do with the challenges specific to a key ingredient: live, weakened flu virus.
“Every year that it’s been available, I have found out where it’s being sold and driven my whole family to get FluMist because we know that when it works, it works,” she says.
How to approve new shots
During the summit, Fauci and the other panels flagged the challenge of testing any new vaccines in the context of widespread exposure to COVID. It’s much harder to know how effective any vaccine candidate is when most potential trial subjects have already been sick or immunized.
The National Institutes of Health, among others, are working on determining what are called “correlates of protection,” or markers of a renewed immune response in blood, saliva, or elsewhere, to measure how strongly a person responds to a vaccine. The correlates are already used to select each year’s flu vaccine.
“I think one of the most important and really pressing issues is identifying correlates of protection that regulatory agencies would be willing to accept for approval,” said Reddy. “What’s the goalpost we need to hit? If that was known, that would make it very easy to design those trials.” That’s a point that Food and Drug Administration advisors have raised in recent meetings as well, and will likely come up again as these COVID vaccine discussions continue through 2022.
