
Gregory Breed doesn’t remember exactly when he began suffering from symptoms of a chronic prostate infection. The 62-year-old estimates they began when he was in his mid-20s. By his early 30s, he was taking painkillers for lower back pain, a common outcome of the disease. Over the years, his doctors put him on course after course of oral antibiotics: trimethoprim-sulfamethoxazole and levofloxacin. But a few months after he stopped taking the drugs, his infection would return.
Prostate infections or prostatitis, one of the most common urinary tract issues in adults, are notorious for recurrence as antibiotics often can’t penetrate tissue deep enough to kill all the bacteria. Over time, with repeated exposure to medicine, the E. coli causing Breed’s infection was becoming increasingly drug-resistant, meaning the bacteria developed the ability to evade and survive treatment. To make matters worse, Breed’s immune response was growing more severe. Between 2000 and 2010, he was hospitalized with sepsis, a life-threatening condition caused by the immune system’s overreaction to an infection, at least five times. Each time he would recover, only to battle another flare-up.
In 2018, Breed began feeling the familiar symptoms of prostatitis. He drove to his local health facility in Casper, Wyoming, where his doctor prescribed a new oral antibiotic: fosfomycin. Six months later, the E.coli in his prostate had developed resistance to that treatment too. It was the last drug that Breed could take orally; with no other options remaining, his doctors installed a catheter in his arm and taught him to administer intravenous antibiotics to himself.
Breed started his first round of IV antibiotic treatment, but the bacteria causing his infection quickly became resistant to that and then to a second IV antibiotic. When his infection came back the next time, Breed’s physician prescribed a third IV antibiotic that Breed fed into his veins three times a day. The new drug was only temporarily effective at reducing the E. coli in his prostate and couldn’t cure the infection. Breed’s physician estimates that his patient was on a different antibiotic regimen for some six months out of each year.
“Greg was constantly in the practice,” Ismail Jimada, Breed’s doctors between 2018 and 2022, says. “We were running out of solutions.”

Breed routinely maxed out his health insurance, paying thousands of dollars out of pocket for his medical bills. The infections took a toll on his loved ones and work life. “I have a very supportive family, but it was hard,” Breed recalls. “There were a lot of years there I didn’t get to be around my family near what I would have liked to.” Some days he struggled to get up and manage his small business selling pipes for oil wells. With limited options for medical care in rural Wyoming, he sought out infectious disease specialists across the country. But with the E. coli in his prostate already resistant to many go-to antibiotics, they didn’t have many treatments to offer him.
Breed’s infection was just one of more than 2.8 million cases of drug-resistant infections recorded by the Centers for Disease Control and Prevention in the US each year. Illnesses that defy antibiotics, antifungals, or other antimicrobials are challenging, if not altogether impossible to treat. More than 35,000 Americans die from drug-resistant infections annually. Globally, that number likely exceeds one million. In 2021 the World Health Organization declared antimicrobial resistance “one of the top 10 global public health threats facing humanity.” Antibiotics are a cornerstone of modern medicine; as pathogens like E. coli , Candida auris, and HIV develop a tolerance to the remedies, common infections, routine dental procedures, and surgeries could become life-threatening. Just in time, however, scientists and physicians are turning to an obscure, decades-old antidote to get drug-resistant infections back under control.
A race to replace antibiotics
Antimicrobial resistance is just another consequence of evolution: Bacteria, fungi, and other microorganisms are constantly adapting unique survival mechanisms to keep up with humans and a changing world. Still, researchers believe the overuse and misuse of antimicrobials in medicine and agriculture is accelerating the emergence and spread of resistance. New drugs capable of killing these evolving microbes could stave off the problem—but it takes millions of dollars and more than a decade of research and development to put out such a product. There hasn’t been a novel class of antibiotics since the 1980s, in part because most major pharmaceutical companies have stopped trying to discover one.
Out of desperation, Breed began scouring the internet for alternative treatments. Sometime in late 2017 or early 2018, he stumbled across a Ted talk by UC San Diego epidemiologist Steffanie Strathdee. In it, Strathdee describes an ill-fated trip to Egypt in 2015 where her husband, Tom Patterson, contracted a drug-resistant infection caused by Acinetobacter baumannii. When they arrived home in California, Patterson fell into a coma; his organs began to shut down. The bacteria behind his infection was already resistant to all known antibiotics.
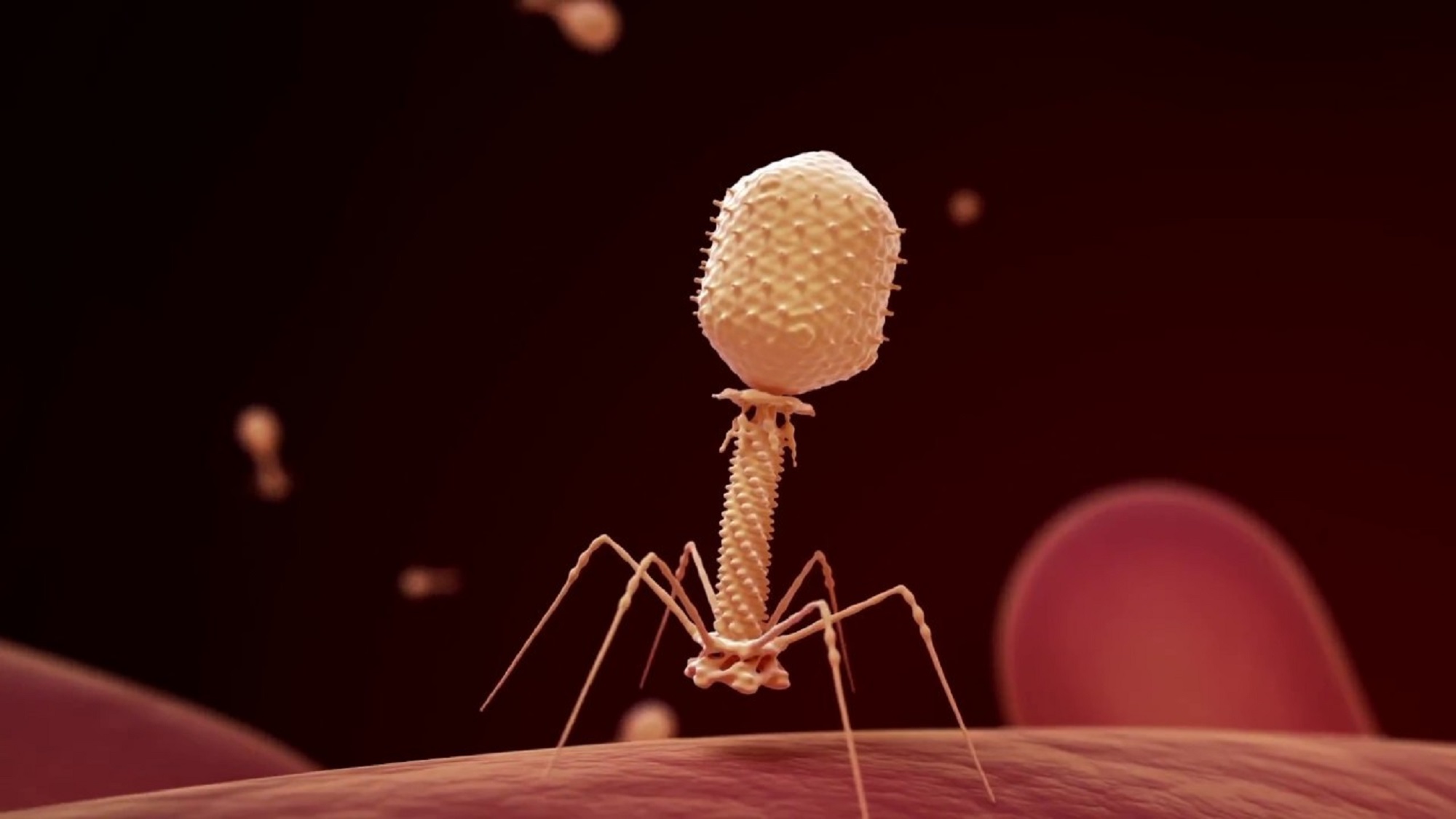
Strathdee also turned to the internet and found a glimmer of hope: a treatment first discovered in 1917 that had been used in the Soviet Union for decades but largely overlooked by the rest of the world. The method taps bacteriophages, a type of virus that infects and kills bacteria, to combat infections. Scientists have long known that there are billions of different bacteriophages, or “phages,” on the planet. They often live in sewers and other germ-filled places and are highly specific, killing only one or sometimes a few strains of bacteria with no documented harm to humans.

Strathdee reached out to scientists publishing phage research at the time. She thought if they could find a virus that could infect and kill the specific A. baumannii strain causing her husband’s infection, then he might have a chance to survive. Researchers at Texas A&M University immediately responded to her urgent e-mail and started hunting for the right type to combat the culprit. After just a few weeks, they found a few candidate specimens and mailed them to California. The treatment would be experimental—there were no known cases of intravenous phage therapy in the US at the time, according to Strathdee—so Patterson’s clinical team requested emergency use authorization from the Food and Drug Administration (FDA). It was granted within a day. At the UC San Diego Medical Center, doctors injected the phages into Patterson’s abdomen, and a few days later, directly into his bloodstream. Less than a week after the treatment began, Patterson woke up, kissed his daughter’s hand, and went on to make a full recovery.
Building a new path
In 2018, Strathdee co-founded the Center for Innovative Phage Applications and Therapeutics, or IPATH, at UC San Diego to further develop phage therapy for drug-resistant infections. Later that same year, Breed e-mailed the group inquiring if method might also work for his chronic prostate infection. The IPATH team got to work sending specimens of Breed’s E. coli to a network of phage researchers around the country, searching for an effective phage match for much of 2019. It was ultimately provided by the TAILOR lab at the Baylor College of Medicine, which creates “customized phage cocktails for no-hope cases when antibiotics and standard of care have failed,” its founder Anthony Maresso says. Then it took just about one day to receive compassionate use authorization from the FDA.

In January 2020, Breed and his wife flew to San Diego to begin the phage treatment, which IPATH provided for free. “I was excited but extremely nervous about it. When you think about putting the substance they tell you the phage is derived from into your body, it’s not a real good thought,” Breed says. (He’s referring to sewage, the most common source for effective phages.) “Especially when they hook you up and say, ‘Okay, we’re gonna watch and see what happens,’ and you know there’s not a whole lot of people ahead of you who have done it. It’s a really scary situation to be in.”
The IPATH team injected phages into Breed’s catheter. For the next two weeks, he continued with the procedure twice a day, according to Saima Aslam, an infectious diseases physician and IPATH’s clinical lead. Breed’s symptoms—pain, fever, and urinary problems—faded. After six months, laboratory tests confirmed there was no detectable E. coli in his prostate. It has remained that way ever since, and Breed has not suffered any adverse effects from the undertaking.
In high demand
After his decades-long battle, Breed was finally cured. So, too, are a growing number of patients with drug-resistant infections. Phage therapy remains experimental in the US and can only be used in emergency or compassionate use cases when few or no other treatments are available. Data describing its effectiveness and safety remains limited: Results from single cases are not always consistently reported, and only a handful of clinical trials have been conducted. What’s more, across the few health centers now providing phage therapy, there are variations in how it’s administered—intravenously, orally, topically, or intranasally—and in patient-selection criteria. Despite these challenges, the emerging body of research consistently concludes that phage therapy is safe.
There are, however, mixed results regarding phage therapy’s ability to cure infections. One review published in 2022 summarized safety and efficacy findings from 13 clinical trials conducted around the world between 2005 and 2021. All the trials, including six tests that administered phages to healthy children and adults to compare them to sick patients, independently concluded that the remedy was safe. Only seven of the 13 studies assessed the clinical outcomes of sick patients who received phages to treat a variety of conditions from leg ulcers to ear infections. Only two consistently reported improvements in patients’ symptoms following phage therapy; the authors of the wider review note this may reflect a poor match between the selected phage and the bacteria it was meant to target.
A separate study published in 2023 reported that 11 of 20 patients in the US who received phage therapy for infections caused by mycobacterium had favorable clinical responses. The IPATH team itself has treated 18 patients, including Breed, with phage therapy in the past five years and has had a success rate of 82 percent, Aslam says. She and other physicians and phage researchers are calling for additional research and clinical trials to better understand phage therapy and set forth best practices. There are more than 20 ongoing clinical trials—many for people with cystic fibrosis or prosthetic joint infections—–registered with the US government now.

The current ad-hoc model of sending samples to researchers across the country in hopes for a phage match often delays time to treatment. It usually takes about four to six months to scour repositories—or sewers—for a good phage match, Aslam explains, which means that patients should be in somewhat stable condition to be a candidate. (The FDA typically approves phage therapy for critically ill patients within 24 hours and for more stable patients within 30 days.) The method is also not sustainable for large-scale use. The US government and several universities around the world are working to compile databases that list their discovered phages and the bacterial strains they target. Several biotechnology companies with private collections have also sprung up in recent years; some researchers are even developing and patenting genetically modified phages that are shelf stable or can target more than one type of infection-causing bacteria.
While there is still a long road ahead, phage therapy is one of the few tested solutions for drug-resistant infections—and could some day be comparable, if not cheaper, to the cost of antibiotics. Aslam envisions that in the future, different phage products will be available at pharmacies just like other medicines. But for now, the therapy is proving lifesaving and life changing for lucky individuals like Breed and Patterson. “I have three young grandkids … I was to the point that I was wondering if I really was gonna enjoy the time with them,” Breed says. While he still has some lingering side effects from such prolonged antibiotic use, in terms of quality of life, “it is a thousand times better than it was” before the phages entered the battle.