Perhaps you think of X-rays as the strange, lightly radioactive waves that phase through your body to scan broken bones or teeth. When you get an X-ray image taken, your medical professionals are essentially using it to characterize your body.
Many scientists use X-rays in a very similar role—they just have different targets. Instead of scanning living things (which likely wouldn’t last long when exposed to the high-powered research X-rays), they scan molecules or materials. In the past, scientists have X-rayed batches of atoms, to understand what they are and predict how those atoms might fare in a particular chemical reaction.
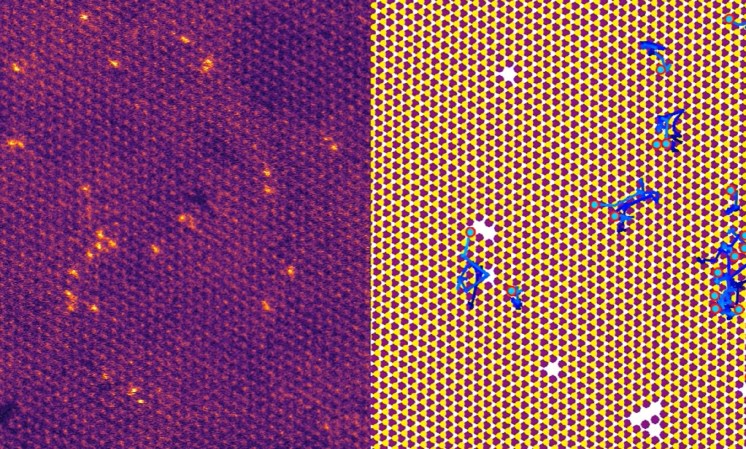
But no one has been able to X-ray an individual atom—until now. Physicists used X-rays to study the insides of two different single atoms, in work published in the journal Nature on Wednesday.
“The X-ray…has been used in so many different ways,” says Saw-Wai Hla, a physicist at Ohio University and Argonne National Laboratory, and an author of the paper. “But it’s amazing what people don’t know. We cannot measure one atom—until now.”
Beyond atomic snapshots
Characterizing an atom doesn’t mean just snapping a picture of it; scientists first did that way back in 1955. Since the 1980s, atom-photographers’ tool of choice has been the scanning tunneling microscope (STM). The key to an STM is its bacterium-sized tip. As scientists move the tip a millionth of a hair’s breadth above the atom’s surface, electrons tunnel through the space in between, creating a current. The tip detects that current, and the microscope transforms it into an image. (An STM can drag and drop atoms, too. In 1989, two scientists at IBM became the first STM artists, spelling the letters “IBM” with xenon atoms.)
But actually characterizing an atom—scanning the lone object, sorting it by its element, decoding its properties, understanding how it will behave in chemical reactions—is a far more complex endeavor.
X-rays allow scientists to characterize larger batches of atoms. When X-rays strike atoms, they transfer their energy into those atoms’ electrons, exciting them. All good things must end, of course, and when those electrons come down, they release their newfound energy as, again, X-rays. Scientists can study that fresh radiation to study the properties of the atoms in between.
[Related: How scientists managed to store information in a single atom]
That’s a fantastic tool, and it’s been a boon to scientists who need to tinker with molecular structures. X-ray spectroscopy, as the process is called, helped create COVID-19 vaccines, for instance. The technique allows scientists to study a group of atoms—identifying which elements are in a batch and what their electron configurations are in general—but it doesn’t enable scientists to match them up to individual atoms. “We might be able to see, ‘Oh, there’s a whole team of soccer players,’ and ‘There’s a whole team of dancers,’ but we weren’t able to identify a single soccer player or a single dancer,” says Volker Rose, a physicist at Argonne National Laboratory and another of the authors.
Peering with high-power beams
You can’t create a molecule-crunching machine with the X-ray source at your dentist’s office. To reach its full potential, you need a beam that is far brighter, far more powerful. You’ve got to go to a particle accelerator known as a synchrotron.
The device the Nature authors used is located at Argonne National Laboratory, which zips electrons around a ring in the plains of Illinois, two-thirds of a mile long. Rather than crashing particles into each other, however, a synchrotron sends its high-speed electrons through an undulating magnetic gauntlet. As the electrons pass through, they unleash much of their energy as an X-ray beam.

The authors combined the power of such an X-ray beam with the precision of an STM. In this case, the X-rays energized the atom’s electrons. The STM, however, pulled some of the electrons out, giving scientists a far closer look. Scientists have given this process a name that wouldn’t feel out of place in a PlayStation 1 snowboarding game: synchrotron X-ray scanning tunneling microscopy (SX-STM).
[Related: How neutral atoms could help power next-gen quantum computers]
Combining X-rays and STM isn’t so simple. More than simple technical tinkering, they’re two separate technologies used by two completely separate batches of scientists. Getting them to work together took years of work.
Using SX-STM, the authors successfully detected the electron arrangement within two different atoms: one of iron; and another of terbium, a rare-earth element (number 65) that’s often used in electronic devices that contain magnets as well as in green fluorescent lamps. “That’s totally new, and wasn’t possible before,” says Rose.
The scientists believe that their technique can find use in a broad array of fields. Quantum computers can store information in atoms’ electron states; researchers could use this technique to read them. If the technique catches on, materials scientists might be able to control chemical reactions with far greater precision.
Hla believes that SX-STM characterization can build upon the work that X-ray science already does. “The X-ray has changed many lives in our civilization,” he says. For instance, knowing what specific atoms do is critical to creating better materials and to studying proteins, perhaps for future immunizations.
Now that Hla and his colleagues have proven it’s possible to examine one or two atoms at a time, he says the road is clear for scientists to characterize whole batches of them at once. “If you can detect one atom,” Hla says, “you can detect 10 atoms and 20 atoms.”