
Today, the United States Food and Drug Administration (FDA) approved a historic treatment for sickle cell disease. Casgevy is the first medicine based on CRISPR gene-editing technology. The procedure was approved for use in the United Kingdom in November.
[Related: The UK becomes the first country to approve CRISPR treatment.]
“Sickle cell disease is a rare, debilitating and life-threatening blood disorder with significant unmet need, and we are excited to advance the field especially for individuals whose lives have been severely disrupted by the disease by approving two cell-based gene therapies today,” Doctor Nicole Verdun, director of the Office of Therapeutic Products within the FDA’s Center for Biologics Evaluation and Research, said in a statement. “Gene therapy holds the promise of delivering more targeted and effective treatments, especially for individuals with rare diseases where the current treatment options are limited.”
The life-long genetic condition is caused by errors in the genes for the protein hemoglobin. Red blood cells use hemoglobin to carry oxygen through the body. The abnormal hemoglobin makes the blood cells crescent-shaped and hard. The misshapen cells then clump together and block the flow of oxygen to the organs, which causes extreme pain. The cells can then die off early, which leads to anemia.
Sickle cell disease is particularly common among people with Caribbean or African ancestry and affects more than 100,000 Americans. Previously, the only known cure for sickle cell disease was a bone marrow transplant from a donor. These procedures come with the risk of rejection by the patient’s immune system and finding a matching donor is a difficult process.
How Casgevy works
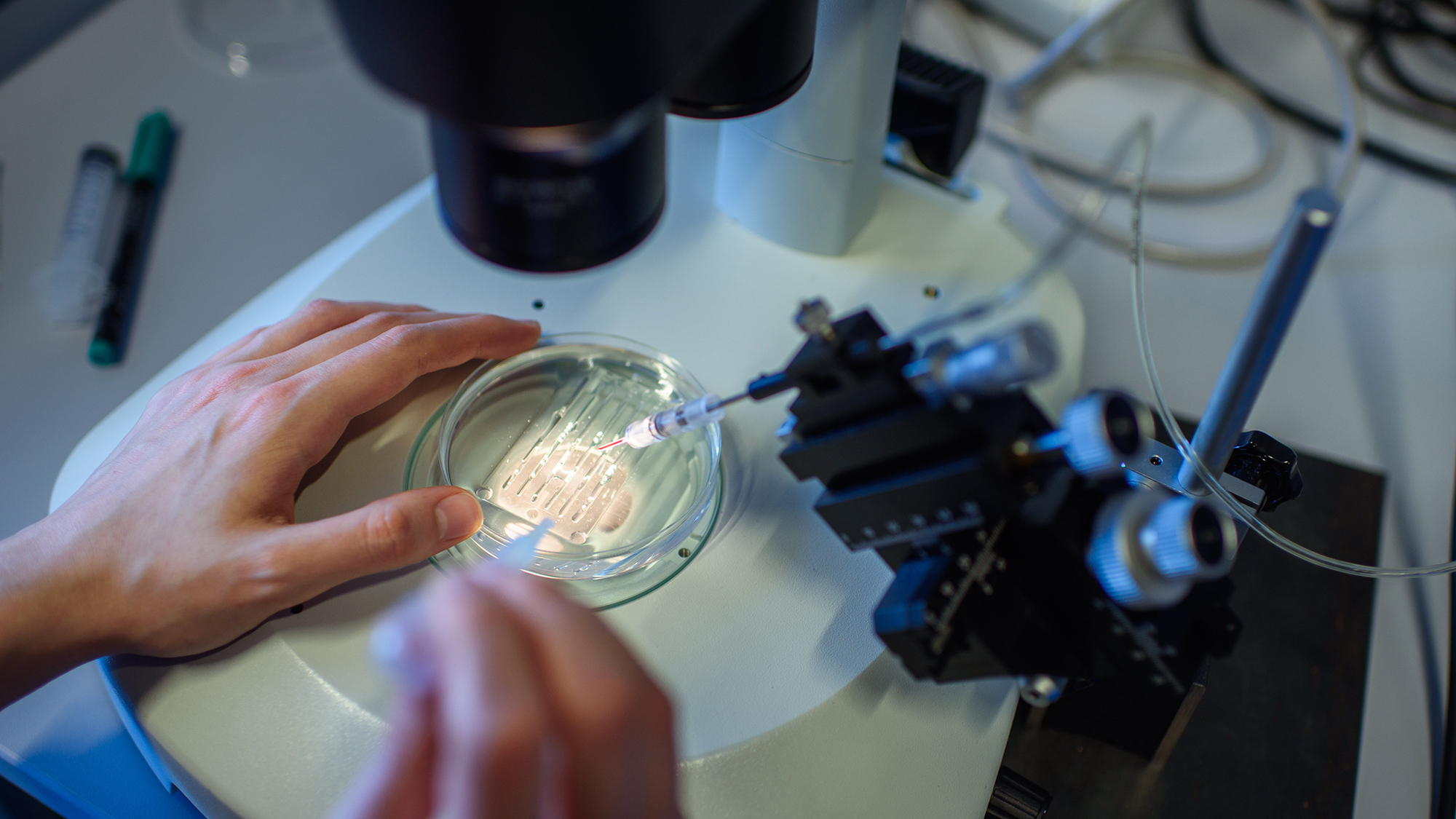
Casgevy uses CRISPR-Cas9 gene editing technique, which allows scientists to make precise alterations to human DNA. French microbiologist, geneticist, and biochemist Emmanuelle Charpentier and American biochemist Jennifer A. Doudna shared the 2020 Nobel Prize in Chemistry for their work developing the technique.
The procedure uses stem cells taken from a patient’s bone marrow. The cells are then brought into a lab and the genes that are meant to switch on a functioning version of hemoglobin are edited with CRISPR. Patients must then go through a “conditioning treatment.” This can involve taking a drug that suppresses the immune system, radiotherapy, or chemotherapy to get the body ready for an infusion of CRISPR-modified cells back into the body. The new treatment does not come with the risk of graft versus host disease the way that a traditional bone marrow transplant does.
The approval comes on the heels of a clinical trial with 45 patients. Of these, 29 patients have been in the trial long enough for the researchers to gauge how effective Casgevy is. For at least 12 months after treatment, 28 were free of severe pain caused by the disease.
[Related: These organisms have a natural gene-editing system that could be more useful than CRISPR.]
How Lyfgenia works
The FDA also approved another sickle cell treatment called Lyfgenia. The gene therapy from Bluebird Bio. also modifies a patient’s stem cells. Both Lyfgenia and Casgevy require a conditioning treatment that removes cells and bone marrow so that they can be replaced with the modified cells.
According to the FDA, patient’s blood stem cells are then genetically modified to produce HbAT87Q. This gene-therapy-derived hemoglobin functions similarly to hemoglobin A, or the normal adult hemoglobin produced by those who do not have sickle cell disease. Red blood cells that have HbAT87Q come with a lower risk of sickling and occluding blood flow. The modified stem cells are then delivered to the patient in a one-time, single-dose infusion.
Lyfgenia’s safety and analysis is based on data from a 24-month study. Of the 32 patients in the study, 88 percent did not have a recurrent vaso-occlusive crises. These hallmarks of the disease occur when blood microcirculation is obstructed by sickled red blood cells, injuring organs and causing extreme pain. It does have a risk of blood cancer and a black box warning is included in the label. The FDA also advises that patients should have continued monitoring for blood cnacer
Questions for the future
These groundbreaking therapies are expected to be incredibly expensive. A report from a fair prices nonprofit the Institute for Clinical and Economic Review, a nonprofit group that helps evaluate the value of medical tests and treatments found that the therapy could cost about $2 million per patient. It could be out of reach for many families and does not include additional care costs.
“We really have to make sure that it is accessible,” Cleveland Clinic pediatric hematologist-oncologist Rabi told NBC News. “This could be an equalizer for people with sickle cell because many patients cannot pursue career options because of the illness.” Hanna previously served on the advisory board for Vertex, who makes Casgevy.
The treatments are also very time consuming. Between the pre-treatment conditioning, treatment itself, and follow up, patients have to spend weeks and possibly months in the hospital.
“I think there will be immediate uptake in some portion of the patients who have more severe disease—frequent pain episodes that cause hospitalization and require opioids for the treatment of pain,” said Boston Children’s Hospital hematologist and oncologist David Williams, told Stat. He added that there could be “bumps in the road” if pharmaceutical companies run into trouble manufacturing these complicated treatments or if the price tag keeps it out of reach for many patients.
The long term effects are also still not known. The FDA says that patients who received Casgevy or Lyfgenia will be followed in a long-term study to evaluate safety and effectiveness.
