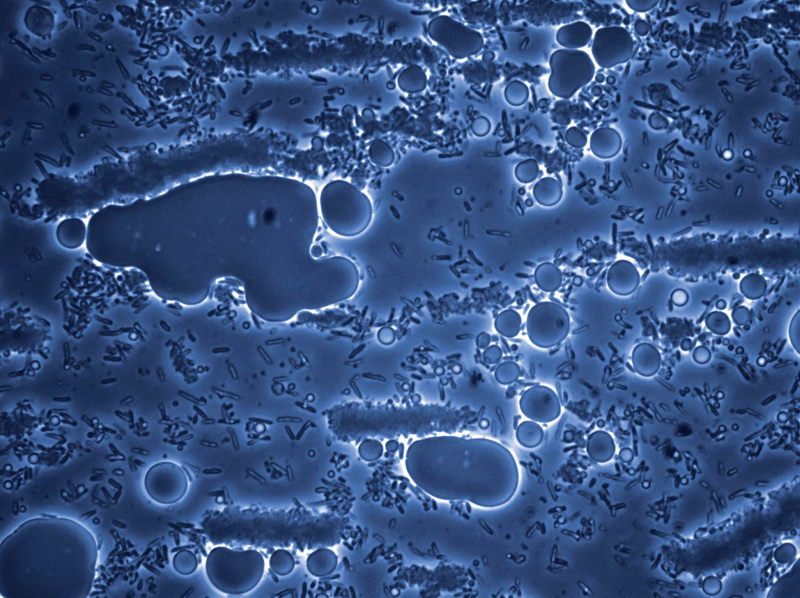

It could be an aerial photo of an oil spill: liquid spheres pooling, oozing, dwarfing a bedraggled landscape. I half expect to zoom in on poisoned seal pups or waterbirds dragging their oil-soaked feathers. But the scene is microscopic. The “landscape” is made of E. coli. And what’s happening is exactly the opposite of what it seems. The little bugs aren’t drowning in fuel. They’re making it.
I’m watching this image on a computer screen at Amyris Biotechnologies in Emeryville, California, where one of the founders, biologist Jack Newman, is giving me a tour. The genetically manipulated E. coli before me are highly crafted units of industrial production, which Amyris is using to turn sugar into novel versions of gasoline, jet fuel and diesel—in other words, the fuels on which the world already runs. Amyris is one of a handful of young biofuel companies putting a brilliant and weird twist on the future of green. It’s betting that, with the help of bacteria, the long-term answer to our gasoline woes will actually be . . . gasoline.
Because as it stands, the main alternative to petroleum, ethanol (a type of alcohol), is fraught with problems. It can’t be pumped through current infrastructure because it tends to corrode pipelines. And according to University of Minnesota economist Jason Hill, even if all the corn grown in the U.S. were converted to ethanol, it would replace only some 12 percent of the 146 billion gallons of gasoline we use every year. Cellulosic ethanol—fuel produced from the cellulosic matter contained in plant stalks and stems rather than from seeds—would solve that problem, but the technology to produce it on a large scale is still a way off. Plus, ethanol simply isn’t as energy dense as petroleum-based fuels.
This is why a growing number of scientists have begun to look to the microbial world for new, environmentally sound ways to make good old-fashioned gasoline. If microbes can be manipulated to turn, say, sugarcane into hydrocarbon fuel—and each new sugarcane crop absorbs most of the carbon dioxide that’s emitted by burning the fuel made from the previous crop—then you’ve got oil-free, nearly carbon-neutral gasoline. It may sound far-fetched, but the evidence is in this picture; oily blobs of hydrocarbons pool around the cells in a pattern that looks like a lava-lamp screensaver. “So this is how you’re gonna save the world?” I ask Newman. “Help save the world,” he corrects.

Radical, but Practical
Newman’s answer is telling. He and his colleagues understand that no single technology is going to solve our energy woes. And joining the army of scientists working on ethanol would be, in the words of their mentor, Jay Keasling, a professor of chemical engineering at the University of California at Berkeley, “like adding another digit to pi.” Instead, Amyris is coming at our petroleum addiction from a different direction: by using synthetic biology—an emerging form of genetic engineering in which microbes are implanted with genes from different organisms—to turn glucose from plant matter into hydrocarbon fuel.
The project rests on a pragmatic realization: Gasoline isn’t going anywhere anytime soon. And in a lot of ways, hydrocarbons work very well. They are easy to pump through pipelines and into gas stations. They’re rich in energy. And today’s gasoline engines are powerful machines finely tuned by decades of engineering and innovation. Why give them up if we don’t have to?
The problem is that while nature is adept at turning sugar into ethanol through fermentation, it’s not so good at turning sugar into hydrocarbons. But as Keasling puts it, “we don’t have to rely on what nature gave us.” He believes we can build something better. And so Amyris has altered nature to carry out fermentation with a twist. By adding genes to bacteria that cause the microbes to create different enzymes, Amyris builds a pathway to carry out the series of chemical conversions necessary to turn sugar into hydrocarbon. They turn a common microbe into a miniature gas pump.
Amyris has done this on an experimental basis. But scaling up these experiments into a partial replacement for the 20 million barrels of oil the U.S. imports each day is a different matter.
The Brewery
Amyris is a distinctly cheery operation. On this afternoon in late November, amid the gene sequencers, microarrays, microscopes, flasks, pipettes and humming refrigerators, rows of miniature pumpkins decorated with smiley faces sit atop tables. Lab coats are embroidered with nicknames like “Soybean” and “Wild Type.” Newman points out that Amyris is almost like a university lab but not quite—”everyone’s too happy.”
As one might expect from a startup, whose entire net worth is pretty much based on its intellectual property, the Amyris guys won’t go into detail about their technical manipulations. Nor will they say what molecules, exactly, they are making. In fact, on the day of my visit, they’re downright cagey. When Newman and I walk through a lab room containing nuclear magnetic resonance machines—which are used to determine chemical structure—and I mention I once worked in a chemistry lab, he gets a little nervous and quickly ushers me into the next room.

But this much we know: Using a device called an electroporator, which uses a brief electrical current to zap temporary holes in a cell wall, it’s possible to add genes from any number of organisms to a cell. Create bacteria with the right genes, and they’ll churn out chemical precursors for everything from pharmaceuticals to food additives to fuel. So to turn these bacteria into tiny fuel factories, Amyris adds genes to E. coli and other microbes that cause them to secrete hydrocarbons after digesting the glucose found in biomass such as sugarcane.
This, of course, is easier said than done. In the Amyris lab I see dozens of young scientists “interrogating” microbes—testing modified organisms to see how well they convert sugar into fuel, then tweaking them some more. The drill, Newman says, is to “think of a bug, build a bug, and test a lot of bugs.”
The testing and tweaking are done mostly through computer analysis of the microbes. As I stand over the shoulder of a biologist named Lance Kizer, he points to his screen and shows me which genes in each bug are switched on, and to what extent. Each gene’s activity appears onscreen as a rising and falling curve. “This is a big part of de-bottlenecking,” Newman says. To make fuel efficiently, you need a microbe that will eagerly convert sugar into the chemicals you want, but that won’t produce unwanted by-products and toxins that could build up and kill the cell. Kizer’s analysis helps determine what genetic alterations can get Amyris closer to its ideal microbe.
Next we move into a large, open room where a small group of scientists is working with flasks that contain modified microbes and sugar. They stir the contents and wait until greasy droplets of fuel float to the surface. Researchers at Amyris perform hundreds of experiments like this every day.
Finally, we reach the “brewery,” a room with exposed copper piping, several steel fermenters and a large vat towering on one side. “Once you’ve got something that works, you come here,” he says. This is where they work on the trickiest part of the process—gauging how the microbes will function on a larger scale. Making the bugs hardy enough to survive the brutal life of a fuel-manufacturing microbe is key. On an industrial scale, microbes are subjected to extreme pressure and high temperatures because so many of the heat-producing bugs are packed together. “When you grow microbes for the production of pharmaceuticals that are high-cost, you can baby them,” says Kinkead Reiling, another co-founder. “With fuel, it’s rough. It’s an old fermenter. You don’t even want to clean the thing because it adds cost. It’s a different world.”
From Pharma to Fuel
Amyris began in 2001, when Newman, Renninger and Reiling were postdocs together in Keasling’s Berkeley lab. At the time, Newman was working in the lab on biosensors, devices that detect the presence of specific molecules. Renninger, who had also done his Ph.D. with Keasling, was focused on bioremediation—using microorganisms to clean up the environment. In the evenings, Renninger, Newman and Reiling would go over to Keasling’s house to brainstorm start-up ideas. (Also present was another postdoc named Vince Martin, whom Renninger calls “the fifth Beatle.”) “We’d bring a bottle of wine apiece and order some bad pizza or Chinese food and drink a fair load,” Renninger says. Over the course of an evening, “the productivity of those meetings went up and up and then slammed to the ground.”
At first, the group considered using algae to make biodiesel. Soon, though, their attention shifted to a project already under way in Keasling’s lab—using synthetic biology to make a cheaper version of a malaria drug called artemisinin. “That technology was just on fire,” Newman recalls. “It was beyond our wildest dreams how well it was working out.” Still, they weren’t sure how to scale up production or bring the drug to market. “That’s where we came up with the idea of a mega-grant from the Gates Foundation,” he says.
The plan worked. In 2003, U.C. Berkeley filed a proposal in conjunction with Amyris and a nonprofit called the Institute for OneWorld Health. A year later, the Bill & Melinda Gates Foundation gave the coalition a $42.6-million grant for work on artemisinin. Amyris agreed to develop the technology on a not-for-profit basis. “For the first year and a half, we just had our heads down, thinking about how we were going to make good on” artemisinin, Newman says. (Amyris expects to deliver its artemisinin-manufacturing technology to a pharmaceutical company this year.)
With artemisinin in progress, the team started thinking about project number two—and this time, they wanted a moneymaker. Amyris’s core technology could be used to make thousands of different molecules, including cheap vitamins, flavors and fragrances. For a while, the group flirted with making cut-rate strawberry flavoring, but the idea failed to really inspire.
That’s when they came back around to biofuels. It was a few months before the movie An Inconvenient Truth came out. Venture capitalists were newly interested in biofuels. So the Amyris team sat down in the office, pulled out scrap paper, and began drawing chemical structures. The goal was to dream up “perfect fuel” molecules—stable compounds that wouldn’t freeze easily and that packed enough energy per molecule to make cost-effective fuels. Some of these molecules happened to look a bit like artemisinin. With candidate structures in hand, they started testing and further modifying bugs to make the new molecules. Suddenly, Amyris was in the energy business.

It Helps To Be a Gambler
Perhaps more than any member of the team, Renninger embodies the collision of environmentalist and capitalist necessary for a project like this—one that’s idealistic, risky and potentially very lucrative. As an undergraduate at the Massachusetts Institute of Technology, he studied combustion engines and air-pollution control. He researched bioremediation and dreamed about someday starting an energy company. But in the meantime, he paid his bills playing blackjack.
In 1994, Renninger joined the notorious MIT blackjack team, which throughout the 1990s took Vegas for millions by counting cards and using statistical analysis. Recruited by one of his fraternity brothers, he began flying to Vegas on the weekends and gambling into the wee hours, sometimes in disguise. On some weekends, Renninger brought in more than $100,000 for the team.
Newman likes to prod Renninger about his days as a card shark. “Ask him how many dumpsters he got thrown in,” says Newman, over dinner at a place called Rubicon in San Francisco. “I didn’t get thrown in any dumpsters,” Renninger says. “I got backroomed a couple of times. But my attitude was, if they break my legs, I’m going to sue and make a lot more money than I ever would playing blackjack.”
I ask him whether he thinks his aptitude for gambling is relevant to Amyris. Newman interjects, “That’s the only reason I joined the group. I was like, ‘Well, at least somebody knows something about money.’ ” Renninger laughs. “It’s calculated risk-taking, right? It certainly makes you more comfortable with risk.” Vegas has plenty of lessons to teach scientists, he says. “The concept of getting back up when you’ve just been beaten down because you know you’re on the right track. There’s plenty of $10,000 bets I lost and followed up with $15,000 or $20,000 bets. It’s that sort of drive that’s pretty common in science. Because sometimes things don’t work.”
Racing the “Gene King”
The number of companies using synthetic biology to produce fuel is still relatively small, but the competition is growing. Oil giant BP, along with DuPont, has begun producing butanol, an alcohol, on a small scale using genetically engineered microbes.
J. Craig Venter, the renegade biologist who in 1998 announced his intention to sequence the human genome using private funds, is also in the game. In 2005 Venter and Nobel laureate microbiologist Hamilton Smith founded Synthetic Genomics, a company that aims to create organisms that can turn plant matter into biofuels. Venter likes to say he’s going from being “the gene king to the oil king.”
His approach to fuels follows his drive to create synthetic life. (In January, he announced that he had produced the world’s first synthetic chromosome, which, when implanted in a cell, would yield the world’s first synthetic life form.) In Venter’s view, it’s better to build new fuel-producing organisms from scratch than it is to genetically manipulate existing microbes.
His plan to make a so-called “minimal” organism before putting it to practical use could slow his progress with fuels, however. “Even if he says he’s created a synthetic organism, it’s going to be many years until it’s usable,” Keasling notes. “The microbes we are creating are usable now.” (Venter counters that he is also working on a jet fuel that uses a modified version of an existing microbe.)
Modifying existing microbes is also the strategy of LS9, a small start-up in San Carlos, California. Founded in 2005, LS9 is, like Amyris, retooling bacteria to produce biodiesel and other fuels (but using different chemical pathways). In addition, it is working on a crude product that would need to be sent to a refinery before it’s usable. LS9’s vice president of research and development, Stephen del Cardayré, says that the company is “actively scaling up” its diesel products and is building a pilot facility in its new lab space to test larger-scale production this year.
Pilot plants will be a major test for all of these companies. “The question of all biofuels is the question of scale,” Venter told me. “It’s an overwhelming scale that we have to produce at. And it’s going to be as much of an engineering challenge as a biology challenge.” For instance, when you’re working on an industrial scale, how do you separate fuel from the microbes that created it? How do you keep the microbes from drowning in the fuel they’re producing?
An even greater question of scale: How can a few small biofuel companies displace the billions of gallons of gasoline that Americans burn each year? LS9’s Cardayré puts it bluntly: “Every one of the companies working in this area could be successful beyond their wildest ambitions, and Exxon would never know it.”
The Next Step
Whether Exxon notices or not, this year Amyris will begin work on a pilot plant capable of producing industrial quantities of fuel. It’s in “advanced talks” with a Brazilian sugar supplier that would give it access to large quantities of low-cost sugarcane and in early-stage talks with Costco and other companies about possible distribution deals. The plan, Renninger says, is to create “a complete line from sugar to consumer” within the next two to three years—first biodiesel, then jet fuel, then gasoline. (Why bio-diesel is going to market first is another Amyris trade secret.)
Of the founders, Reiling is the one who spends the most time on business details, together with CEO John Melo.
“I like to say I’m the realist,” Reiling says. He’s also prone to distraction when asked detailed questions. When I press him to tell me what tests the company plans to run at the pilot plant and where it will be located, he swivels in his chair and, with a slight smirk, asks me if I want to hear a CD called Desolate Messiah, by a German heavy-metal band whose name just happens to be Amyris. “How do you feel about ’80s hair bands?” he continues, slipping the CD into his computer. What follows is a sappy, moody song that could have been a Mötley Crüe reject and that, as far as I can tell, has nothing to do with the pilot plant.
Before I leave, though, he shows me a painting he keeps in his office. It’s of a little boy leaping from one cliff to another across a chasm. The boy’s arms are stretched out. His head juts forward. “He isn’t even looking at the edge. He’s looking beyond the edge,” Reiling says. “He’s 100 percent committed to that jump. That’s what you’ve got to do when you’re going after something.”
Amanda Schaffer is a columnist for Slate magazine and a frequent contributor to the New York Times science section.




