
Apart from their luxury connotation, diamonds are also desired for their far more tangible, useful property—being the hardest material on Earth. The glittering mineral’s durability measures somewhere between 70 and 150 gigapascals (GPa), making it a go-to component for heavy duty drill bits, dentist tools, and spacecraft protective coverings.
Unfortunately, mining and manufacturing diamonds both carry their pitfalls, so materials experts have long sought to synthesize a rival known as carbon nitride—all to no success. After more than three decades of trial and error, however, that losing streak is finally over thanks to researchers collaborating between the University of Edinburgh alongside Germany’s University of Bayreuth and Sweden’s Linköping University.
[Related: A buyer’s guide to ethically sourced diamonds.]
As detailed in a new study published in Advanced Materials, a team led by experts at the University of Edinburgh’s aptly named Center for Science at Extreme Conditions managed to create carbon nitrides—an achievement that has eluded researchers since 1989. As New Scientist explains, measuring between 78 and 86 GPa, the 5-micrometers-wide, 3-micrometers-deep samples are tougher than the world’s second-hardest material, cubic boron nitrade, which usually scores between 50 and 55 GPa. Although the carbon nitride creations confirm part of materials researchers’ early theory (namely, that they can be synthesized at all), the samples do not supplant diamond as the hardest known substance. Because of this, experts now believe diamonds may forever be the hardest material possible.
To make it all happen, researchers first subjected carbon nitride’s carbon and nitrogen precursors to roughly 700,000 times that of the planet’s atmospheric pressure by compressing them between two diamond points. At the same time, lasers heated the precursors to roughly 2732 degrees Fahrenheit. Basically, they simulated conditions only found thousands of miles within the Earth.
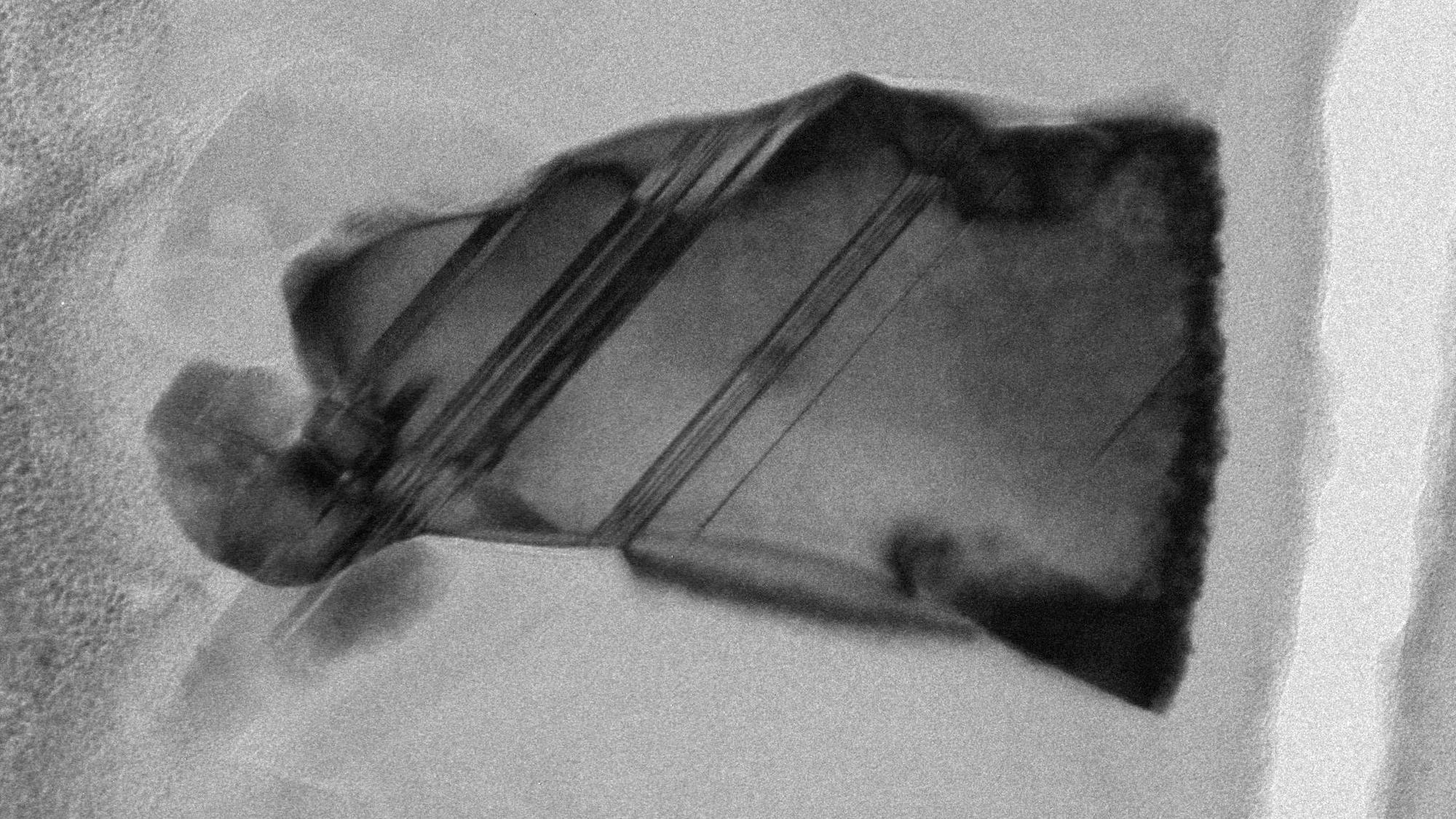
The resultant carbon nitride creations were then assessed using intensely strong X-ray beams at three separate particle accelerators across Europe. In doing so, the team determined three of their synthesized samples contained “necessary building blocks for super-hardness,” according to the University of Edinburgh’s December 14 announcement. Not only that, but the new materials retained their hardness after cooling and returning to normal atmospheric pressure.
“We were incredulous to have produced materials researchers have been dreaming of for the last three decades,” Dominique Laniel, a Future Leaders Fellow at University of Edinburgh’s Institute for Condensed Matter Physics and Complex Systems, said through the school’s announcement. “These materials provide strong incentive to bridge the gap between high pressure materials synthesis and industrial applications.”
After performing additional calculations and experiments, researchers believe synthetic carbon nitrides could also possess photoluminescence alongside a high energy density, meaning very small quantities could be capable of storing comparatively large amounts of energy. To put it a bit more bluntly: carbon nitride could make for some powerful explosives.
Cost is currently the main factor from preventing larger carbon nitride manufacturing efforts. To make bigger samples, researchers need even bigger diamonds to apply the prerequisite pressure—a costly lab tool, to say the least. That said, diamonds can’t generate electrical signals under pressure, and they certainly aren’t known for their explosive properties. Synthetic carbon nitrides may one day become the “ultimate engineering materials to rival diamonds” in many applications, but only if economic and industry viability is ensured, which would take a lot more work.
