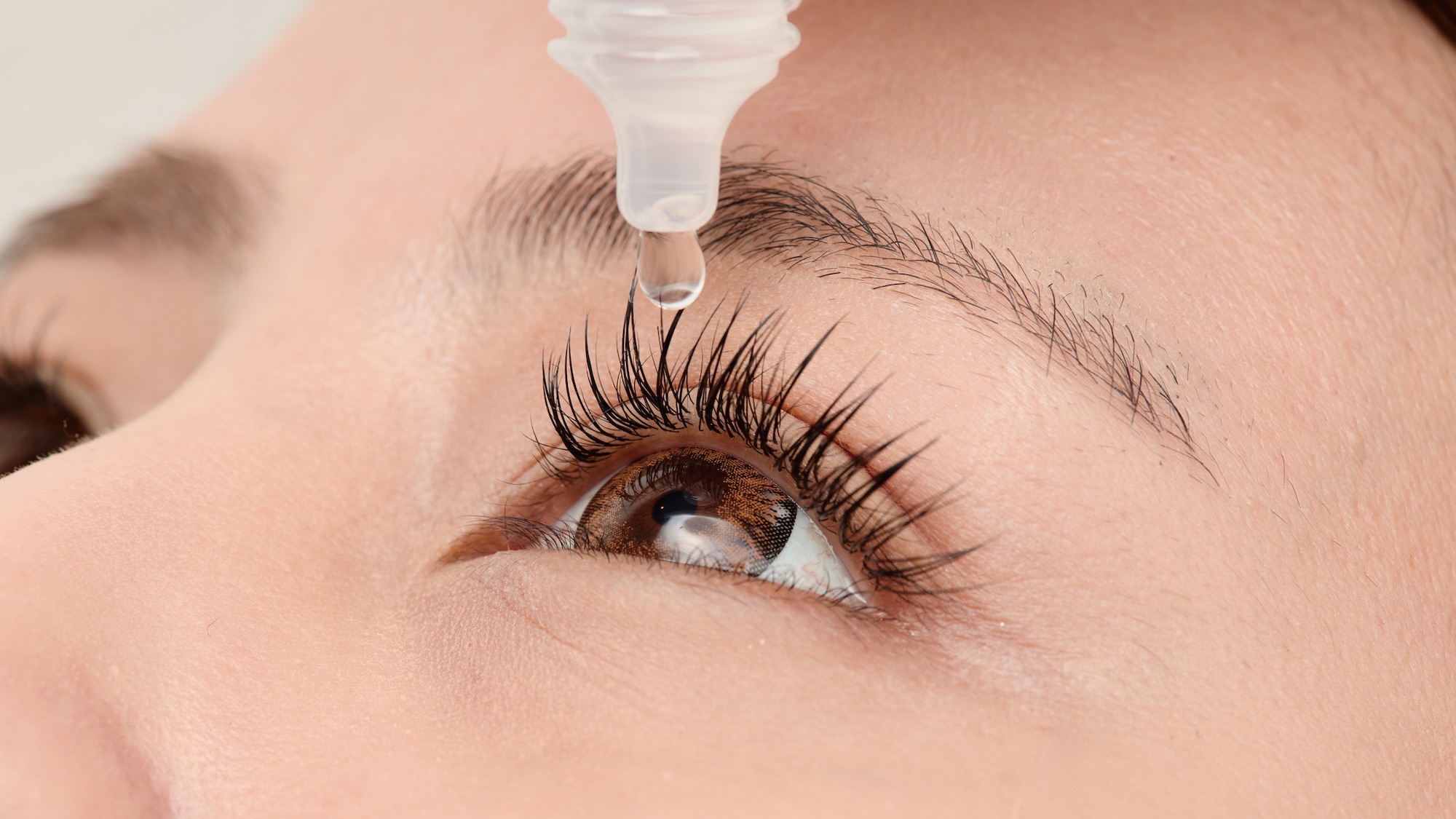

For years, needing reading glasses to correct farsightedness seemed like an inevitable part of aging. This year, the visual accessories might officially be a thing of the past. VIZZ eyedrops by LENZ Therapeutics offer a new tool against age-related farsightedness. The newly approved drops are powerful enough to improve vision by three or more lines on an eye chart within only 30 minutes.
That wide-ranging impact is why Popular Science chose the drops as the 2025 Health category winner. This year’s list also includes ground-breaking improvements to pediatric heart transplants, a potential cure for a deadly blood cancer, and a minimally invasive way to treat prostate cancer.
(Editor’s Note: This is a section from Popular Science’s 38th annual Best of What’s New awards. Be sure to read the full list of the 50 greatest innovations of 2025.)
Grand Award Winner, Health
VIZZ by LENZ Therapeutics: Eye drops correct age-related farsightedness for up to 10 hours at a time
Presbyopia, age-related farsightedness that makes people need reading glasses, affects 128 million people in the US, and close to 2 billion people worldwide. It’s one of the few conditions that is basically guaranteed if you live long enough. Now, an eye drop called VIZZ, developed by LENZ Therapeutics, offers presbyopic patients vision correction for 10 hours at a time.
The aceclidine eye solution got FDA approval for treatment of presbyopia in July. Aceclidine, previously known in Europe as an unremarkable treatment for glaucoma, works on the iris by making the pupil smaller. The smaller the pupil, the greater the depth of focus. In trials that included 1,059 participants, aged 45 to 75, VIZZ improved people’s near vision by three or more lines on eye charts within 30 minutes. Investigators reported that participants could read phones and tablets without reading glasses, and had no loss to their distance vision. Results lasted up to 10 hours.
Previously, other presbyopia drops that worked on a different part of the eye—the ciliary muscle, which is behind the iris—caused brow pain for some users. For users of VIZZ, the most commonly reported adverse reactions are eye irritation, dimming of vision, redness, and headache. The company also recommends consulting an eye care professional before starting these, as miotics like VIZZ could heighten the risk of retinal tears.
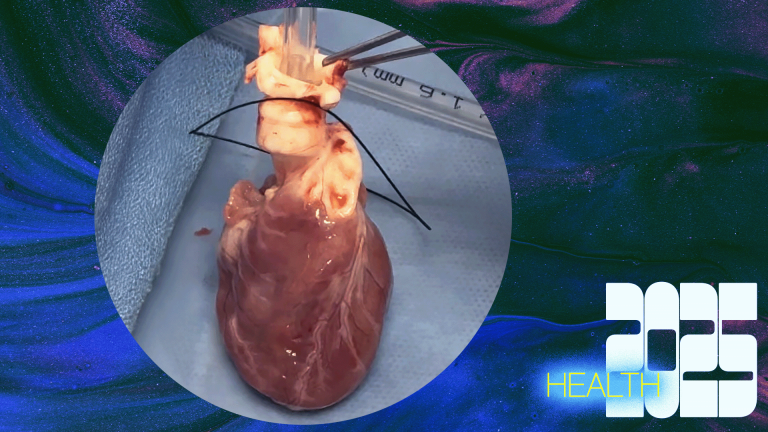
On-Table Reanimation of a Pediatric Heart from Donation after Circulatory Death by Duke University Medical Center: Widening the donor pool for children in need of a heart
Babies are far more likely than adults to die waiting for a heart transplant. In 2022, a study from the Scientific Registry of Transplant Recipients found that more than 1,100 children were on the waitlist, with hundreds more being added every year. Due to a small donor pool and lack of devices usable in pediatric transplants, up to 20% of those children will die while waiting. The most common type of heart donation is donation after brain death (DBD). However, a way to widen the donor pool would be to include heart donations following circulatory death (DCD), or after the donor’s heart stops beating. A known technique called normothermic regional perfusion (NRP) reanimates a DCD heart in order for it to be donated. However, NRP has raised ethical concerns surrounding the definition of death and restoring blood flow to a dead body. As a result, the technique faces bans at many institutions, and viable donor hearts—including pediatric hearts—frequently go unused.
In an attempt to bypass the fierce NRP debate and increase the donor pool for infants in need, a team at Duke University Medical Center developed the on-table reanimation technique, a system with a special circuit that reanimates the DCD heart outside of the body right on the surgical table. Because all of this happens outside the body, the new technique sidesteps many of NRP’s restrictions. Using the new technique, the team successfully transplanted a heart from a 1-month-old donor to a 3-month-old recipient. According to Dr. Joe Turek, a pediatric cardiac surgeon at Duke University, the recipient baby has been healthy and well ever since.
The Duke team is now presenting the technique to colleagues around the country. A wide adoption of it could increase the donor pool for pediatric heart transplants by up to 20% and save countless children’s lives. According to the Duke team, this method could be applied to adult heart transplants as well, offering a less expensive way of getting donor hearts to patients in need.
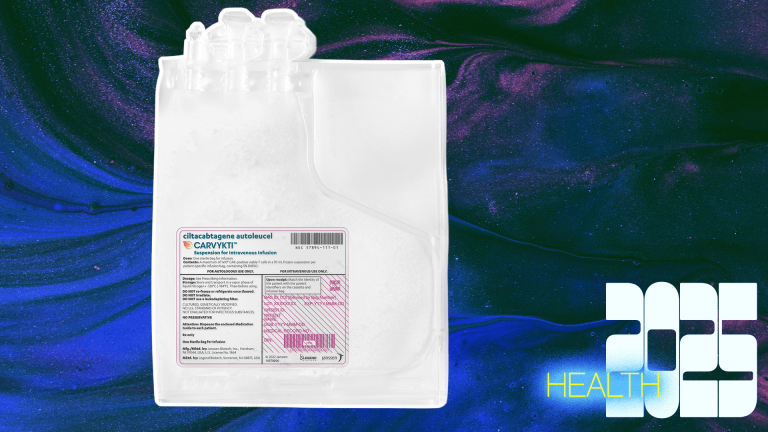
Carvykti by Legend Biotech and Johnson & Johnson: Possible cure for deadly blood cancer
Multiple myeloma has long been considered incurable. The deadly blood cancer, a disease that 36,000 Americans develop each year, eats away at bones, creating holes that weaken the skeleton. In a milestone study published this year, Carvykti, a CAR-T immunotherapy, has yielded long-term remission and survival for multiple myeloma patients. Out of 97 treated patients, one-third had their cancer disappear. This is a striking outcome for people who were facing death after trying everything prior to the treatment. With some patients as of today going on five, or even seven, years post-treatment completely disease-free, researchers are encouraging colleagues to consider using cancer medicine’s forbidden four letter word: cure.
Developed in China by Legend Biotech, which then teamed up with Johnson & Johnson, Carvykti works by extracting a patient’s own white blood cells, retraining them to fight against the cancer, then reinfusing them back into the body. Unsurprisingly, it can be a physically grueling process.
The FDA approved the therapy in 2022, and it’s now causing a stir as follow-up research uncovers its astounding long-term effects. Researchers say the results would likely be even better if Carvykti was used as an earlier line of treatment, and not only as a last resort.
Remote Patient Monitoring program for blood pressure by UC Davis Health: A personalized, widely accessible program to resolve hypertension
Hypertension is a chronic disease that affects nearly half of Americans over age 20, according to the American Heart Association. High blood pressure can put someone at risk of heart disease, heart attack, and stroke. Getting high blood pressure under control can not only lengthen a person’s life, but also improve their ease and enjoyment of everyday activities. UC Davis Health recently pioneered an at-home patient monitoring program using take-home technology to help hypertension patients lower their blood pressure.
The Remote Patient Monitoring program for blood pressure is six months long, but patients can extend their participation in the program for up to a year. The program includes education, medication, and blood pressure cuffs for at-home monitoring. Each patient is given an orientation, group classes, and individual coaching about best practices for their health, all while working remotely with a full medical team. Combined, over 150 patients are either currently in or have gone through the program.
Now, over a year in, UC Davis Health is declaring triumph, citing an average drop in people’s blood pressure from 150/80 mmHg to 125/74 mmHg in only a matter of months, significantly reducing patients’ risk of heart disease. And participants are maintaining their progress even after graduating from the program.
UC Davis Health currently has several remote patient monitoring programs in place and wants to use new technology to make care more accessible. For many reasons—such as distance, age, mobility, or pregnancy—a patient may not be able to easily come in to see the doctor as often as they need to. UC Davis’ model could be useful for rural and urban medical centers alike. According to the program leaders, they are working to not only continue the program, but expand it in years to come.
NanoKnife by AngioDynamics: A minimally invasive intervention for prostate cancer
One in eight men will be diagnosed with prostate cancer in their lifetimes, according to the American Cancer Society. Treatment can include surgery or radiation, but these interventions can damage the nerves surrounding the tumor, leading to complications like erectile dysfunction and urinary incontinence.
Developed by AngioDynamics and cleared by the FDA in December 2024, NanoKnife sends localized electrical pulses directly to the cancerous tissue with a precision that avoids damage to neighboring tissues. Just like some breast cancer patients are given the option of a more targeted lumpectomy instead of treating the entire breast, eligible prostate cancer patients now have a more focused, radiation-free alternative that doesn’t require treating the entire gland.
The NanoKnife System offers men with prostate cancer that hasn’t yet spread a minimally invasive solution with limited quality-of-life side effects before doctors turn to other, more aggressive treatments. It is now being used in hospitals around the country.