Hunting The Elusive Fat Pill
Obesity is booming, yet there are only two medications approved for long-term weight loss. Why is it so hard to make a diet pill that works? For one thing, evolution hates diets
As magic little pills go, the weight-loss drug rimonabant was destined to be huge. It was supposed to put a dent in the obesity epidemic and help people quit smoking and improve their cholesterol along the way. Pharmaceutical execs expected it to usher in a new class of drugs bigger than cholesterol-controlling statins, like Lipitor, Pfizer’s $1-billion-a-month blockbuster. Such was the promise when rimonabant hit Europe in 2006 under the brand name Acomplia.
But the drug never made it to American medicine cabinets. Doctors soon realized that rimonabant exacerbated already high rates of anxiety, depression and suicidal thinking among the obese. Last fall, manufacturer Sanofi-Aventis pulled Acomplia from shelves after five people from a trial of 36,000 patients taking it committed suicide (as did one person taking a placebo). The company abandoned the drug, and concerns about the compound’s safety led Merck and Pfizer to dump their copycat versions, both in late-stage trials. “The class as a whole is defunct,” says Nick Turner, an industry analyst with Mirabaud Securities, an investment firm that tracks pharmaceuticals.
In And Out
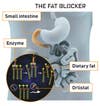
Now doctors are back to square one, with precious few medicinal options for the two thirds of Americans who are overweight. For the one third who are obese, the situation is dire. Obesity, defined as having a body-mass index of 30 or greater, sets the stage for diabetes and killer conditions such as heart disease, stroke and cancer. Aside from advice (stop eating) and stomach surgery for the severely obese (say, 5-foot-8, 265 pounds), there are just two anti-obesity medications approved for long-term use, and the pounds come right back on as soon as you stop taking them. The appetite suppressant sibutramine, a non-addicting cousin of amphetamines, can increase blood pressure in an already hypertensive population, and the fat blocker orlistat (sold over the counter as Alli) can cause what doctors call “fecal urgency” and diarrhea. Worse, both produce only marginal weight loss. “That’s the real killer,” says Steve Bloom, who heads the metabolic-medicine department at Imperial College London. “The reality is, we seem to be getting nowhere.”
The same reasons it’s hard for most people to slim down in any permanent fashion through diet and exercise account for the fact that we still don’t have an anti-obesity drug that’s safe, effective and tolerable. But there is hope in the pipeline. A handful of candidates stand to deliver weight-loss figures big enough to improve not just health but body image, a key to getting patients to stay with the treatment. Bear in mind, the studies are still small—usually only several hundred volunteers—and short, meaning there isn’t enough statistical power to spot potentially dangerous side effects. So we’re not picking any winners here. But they all control appetite surprisingly well, and they do it through very different methods, so it may not be one pill that keeps us thin but a cocktail of them. “I think the future is going to be about hitting multiple pathways with multiple agents,” says Ken Fujioka, director of nutrition and metabolic research at the Scripps Clinic Center for Weight Management in La Jolla, California.
Biology Won’t Budge
If chemically enhanced weight loss sounds a bit too Hollywood for you, consider what we’re up against. The human body is engineered to hoard calories in order to survive famine, the primary threat to human existence until only recently. Start to lose weight, and your brain sets off alarm bells, initiating mechanisms to counteract the trend and override your most ardent efforts to eat less. This biological drive to store fat turns out to be a perverse failsafe in the modern industrialized world, where food is plentiful and packed with calories. When you pit willpower against biology, guess which loses out? “The problem is that once you’re obese, your brain doesn’t know it’s obese,” says neurologist Barry Levin, an obesity researcher at the VA Medical Center in East Orange, New Jersey. “When we put people on a diet, it doesn’t matter how fat they are; they conserve energy, and their brain says store energy. Nobody ever planned for people to get obese.”
The result of the colossal mismatch between our bodies and our environment is that, these days, to be overweight is to be normal. The World Health Organization predicts that obesity numbers will increase from 400 million today to 700 million in 2015. All those overweight contestants on The Biggest Loser are, evolutionarily speaking, big winners—by virtue of their existence.
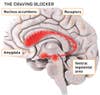
Reward Center
So how do we override our most ingrained survival system? Ill-fated rimonabant attempted to do it by blocking signals that make you feel blissful after satisfying a craving, an important reward mechanism that reinforces the drive to eat. Carried by hormones called endocannabinoids, these feel-good messages are responsible for, among other things, marijuana’s tendency to make people hit Taco Bell at 3 a.m. By reverse-engineering “the munchies,” scientists were able to create a drug that did just the opposite—control appetite, and thus food intake.
But it also reverses the desirable effects of endocannabinoids, dangerously suppressing mood as well as our appetite for more than just food. “The reward system is involved in eating and pleasure and sex and happiness,” says University of Liverpool psychologist Jason Halford, an expert on eating behavior. “If you can’t demonstrate that your drug is only affecting the reward for food, then you’re in trouble.” The receptors for endocannabinoids are scattered throughout the body in the millions; they’re found in the spinal cord, fat cells, brain and other organs. It’s no surprise, Halford says, that there were side effects. Or as Bloom puts it, “What a dumb idea.” If aiming at one widespread receptor doesn’t work, what will?

Mental Block
Triple Threat
Danish researchers recently decided to take aim at the brain chemicals serotonin, norepinephrine and dopamine, after noticing that an experimental treatment for Parkinson’s and Alzheimer’s disease caused patients to spontaneously lose weight. A complex network of cellular brakes and accelerators determines what, when and how much we eat. At the hub of this feedback system sits the hypothalamus, the brain’s appetite center. It coordinates input from other areas of the brain with signals from the body, including hormones produced by the stomach, pancreas and liver, for instance, that inhibit or stimulate appetite. Neurotransmitters are key governors of this system, and the drug tesofensine increases levels of all three.
We’ve known for decades that serotonin influences appetite, and in fact, sibutramine, a hunger suppressant approved by the Food and Drug Administration in 1997, targets serotonin as well as norepinephrine. But it elicits a weight loss of only about 4 percent before patients plateau. Which brings us back to the dilemma of losing weight without triggering our survival response. “How would you build a machine that would ensure you would eat when your energy stores are low?” asks Levin, a leading researcher in the neural circuitry of metabolism. “You would build in a huge amount of redundancy to make sure that the machine eats. Every time you interfere with one of those systems, another one kicks in.” The hope with tesofensine is that adding the neurotransmitter dopamine to the mix might override some of this redundancy. And early indications suggest that the effect may be more than incremental.
The researchers compared different doses of tesofensine, licensed by the Danish company Neurosearch, against a placebo in a group of 203 obese patients. All the participants went on an exercise-restricted diet (typical in such trials), and after six months, the patients taking tesofensine had lost far more weight than the placebo group—12.6 percent versus 2 percent at the maximum dose. Even if you subtract the loss attributable to placebo (that is, diet alone), the resulting 10.6 percent is more than twice that with sibutramine, not to mention the “miracle” pill rimonabant. For the average person, that turned out to be 23 pounds. Louis Aronne, past president of the Obesity Society, who is not involved with the tesofensine trials, calls the results “very exciting.”
Like sibutramine, tesofensine increases heart rate by between five and nine beats per minute, which may or may not prove dangerous. Unlike sibutramine, however, the medium dose, which still elicited an average of 11.2 percent weight loss including the placebo effect, didn’t increase blood pressure, and that’s a key hurdle for someone with an elevated risk of heart disease. The other side effects were dry mouth, nausea, constipation, diarrhea and insomnia. On the upside, there was no increased risk of depression. Even more significant, the study authors didn’t exclude patients with a history of anxiety or depression—as Sanofi-Aventis did in the marquee rimonabant trials—making it less likely that they would uncover unexpected side effects in the general population.
Still, the authors are the first to admit that the numbers are small, and they will have to look at more patients over a full year to really assess the side effects. (A larger, Phase III trial is in the works.) And it’s worth pointing out that these neurotransmitters have a major influence over a wide range of emotions and behaviors, as do endocannabinoids. “I’d love to see what people are like on this drug,” Halford cautions. “I would be very surprised if it only affected appetite.”
One-Two Punch
There’s no question that Empatic affects more than appetite. It’s a proprietary combination of two drugs approved for other uses: zonisamide, an anti-seizure medication, and bupropion (known as Wellbutrin and Zyban), an atypical antidepressant that was also approved for smoking cessation in 1997. Again, the discovery of the weight-loss aspects of these drugs was accidental, and in the case of zonisamide, it was considered a drawback in an early trial of epilepsy patients. While both drugs cause minor weight loss of their own accord, neither one has proved effective for long-term treatment of obesity, because weight-loss plateaus after about six months.
What’s disconcerting about zonisamide is that although we know that it increases serotonin and dopamine, no one is sure precisely how it works, or the full extent of its effects in the brain. As for bupropion, it increases levels of norepinephrine and dopamine. One hint of how the combination packs more of a wallop than either one individually is that the two drugs complement each other on some level; Burroughs Wellcome (now GlaxoSmithKline) had to reintroduce bupropion at a lower dose in the 1980s because a number of patients had seizures.
The leading theory on Empatic is that it stimulates reciprocal pathways in the hypothalamus that regulate appetite and energy expenditure. Researchers suspect that part of this drug combination stimulates neurons that send appetite-reducing signals, and the other part shuts off some of the alarm bells that go off when energy stores drop. “You’re stopping these internal brakes to the weight-loss process,” explains endocrinologist Frank Greenway. As director of the Pennington Biomedical Research Center’s outpatient clinic in Baton Rouge, Louisiana, Greenway has conducted some of the Empatic trials for manufacturer Orexigen Therapeutics, a pharmaceutical company in La Jolla that focuses exclusively on obesity treatments.
According to Orexigen, which reported the results of a yearlong Phase II trial at the Obesity Society’s annual meeting last fall, volunteers taking the highest-dose combination lost 14 percent of their body weight. Shedding 42 pounds off a 300-pound frame may not sound very dramatic, but it’s more than enough to significantly improve mood and energy levels and slash cholesterol, high blood pressure and the risk of contracting type-2 diabetes. In patients who already have diabetes, losing that kind of weight can make their disease more manageable. In light of these benefits, the side effects for zonisamide alone seem tolerable—dizziness, drowsiness and headache—and so far there are few reports of depression or anxiety.
If these kinds of numbers stick through Phase III trials, well, let’s not forget the lesson of rimonabant. It’s dangerous to tinker with brain pathways affecting emotions without knowing the full extent of those effects, especially when putting someone on a drug for life, points out behavioral physiologist Mark Friedman, associate director of the Monell Chemical Senses Center in Philadelphia. Big Pharma’s current approach, he says, “is the equivalent of messing with your visual cortex to correct nearsightedness, instead of wearing glasses.” Rather than “parachuting into the brain,” he adds, we should look at the hormones and neural pathways that regulate appetite physiologically. Signals from the body tell the brain you’re full, and alterations in these signals seem to be responsible for the long-term effectiveness of bariatric surgery, a technique to reduce stomach size. It’s the only obesity treatment proven to cure diabetes, increase life span, and reduce heart disease and cancer rates—which is why a number of companies are cooking up compounds that work on the same principles.
Body Blow
Putting on weight can feel like a one-way street for most people, because the biological signs are all pointing in the same direction: eat! That drive has a lot to do with leptin, a hormone produced by fat cells, whose purpose is to inform the brain how much energy is in the reserve tanks. If leptin levels drop, your brain kicks into foraging mode to stock back up on calories. If they rise, the brain knows you’re full. When leptin was discovered in 1994, scientists thought they had found the keystone to reversing obesity—just crank up leptin, and people would eat less. In fact, that only works in obese people with a rare genetic deficiency of leptin.
In genetically healthy adults, further studies revealed that obese people already have huge amounts of leptin coursing through their veins. It turns out that the brain becomes resistant to the hormone if there are too many fat cells pumping it into the system. It’s only when leptin is missing that the brain really puts up a fuss—in other words, whenever you go on a diet. It’s another example of our biology guarding against starvation, but not so much against obesity.
The worst part of the leptin equation is that when your body weight drops by about 10 percent, metabolism drops to conserve energy, fat oxidation drops, and your muscles become about 40 percent more efficient and burn fewer calories. “That’s the double whammy,” Aronne says. “Energy expenditure is reduced, and you lose your sense of fullness.” So you want more, even though your body needs less.
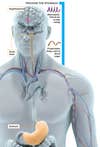
Gut Reaction
The San Diego–based biopharmaceutical company Amylin may have found a work-around. Its plan is to use a combination of metreleptin, a synthetic version of leptin, and pramlintide, a synthetic version of another hormone secreted by the pancreas (called amylin) to keep your appetite in check. Metreleptin delivers a long-term signal, and pramlintide provides a short-term signal that makes you feel full after individual meals. So, with the combination, pramlintide helps you lose the weight because you eat less at each meal, and metreleptin helps you keep it off because your brain isn’t panicking about low leptin levels. Scientists at Amylin are unsure how this overcomes leptin resistance, but they know that it works. In one six-month study published, patients lost 12.7 percent of their body weight.
Even more reassuring, Fujioka says, is that “we know exactly where the compounds go and what they do.” Which is to say, a lot of researchers believe it’s safer to interfere with signals going to the brain than those pinging around within the brain. And if you look even further down the pipeline than these three experimental treatments, the number of trials to manipulate various hormones suggests that this tack may turn out to be the future of anti-obesity treatments.
What’s the downside? Aside from nausea, mainly needles. Hormones must be injected, twice a day in the case of Amylin’s compound, because in pill form the stomach would simply digest them. For people with diabetes—many of whom are obese and already familiar with giving themselves injections—that’s no big deal. But what about all those overweight people unaccustomed to daily needle pricks?
Kirsten Raun, a researcher for the Danish company Novo Nordisk, which is developing a diabetes drug called liraglutide that could also treat obesity, doesn’t believe needles are an obstacle. “We used to think that it would be impossible to go with injections for obesity patients, but actually it seems like they will do anything to lose weight,” she says. “When we had ads in the paper to recruit people for a trial, they were queuing up around the block.”
Desperate Measures
For those who have never been overweight, it can be hard to accept the notion that obesity is a medical problem and not a failure of will. But obviously, nobody wants to be fat, any more than they want to have high cholesterol. Yet it wasn’t that long ago that the prevailing treatment for cholesterol was a heavy dose of advice: Change your lifestyle. Cutting out eggs and butter and saturated fats certainly improves cholesterol, but not to the degree that statins do. The fact is, cholesterol drugs save lives. Likewise, diet and exercise are crucial elements of losing weight, but many researchers agree that today they’re just not enough.
In the wake of rimonabant, the hormone option seems the most palatable and plausible. Perhaps these master signals will be strong enough to override our deep-seated biological drives. But, as the psychologist Halford points out, there is no one mechanism in the body responsible for obesity, and it’s a frustratingly complex problem. He knows this on a personal level, too. Standing 5-foot-7, Halford has, over the past few years, pushed the scales up to 178—about 20 pounds overweight. “Largely because of traveling a lot and alcohol consumption,” he admits. “It just kind of crept up on me.”
Eric Hagerman is a contributing editor at POPULAR SCIENCE. His most recent article, in February, was about Swiss pilot Yves Rossy and his adventures flying a jet-powered wingsuit.