
Excerpted from WHY WE DIE: The New Science of Aging and the Quest for Immortality by Venki Ramakrishnan with permission from William Morrow, an imprint of HarperCollins. Copyright © 2024 by Venki Ramakrishnan.
Lessons from a Lowly Worm
We all know families of long-lived individuals. But exactly how much do genes influence longevity? A study of 2,700 Danish twins suggested that the heritability of human longevity—a quantitative measure of how much differences in genes account for differences in their ages at death—was only about 25 percent. Further, these genetic factors were thought to be due to the sum of small effects from a large number of genes, and therefore difficult to pinpoint on the level of an individual gene. By the time that the Danish study was carried out in 1996, a lowly worm was already helping to overturn that idea.
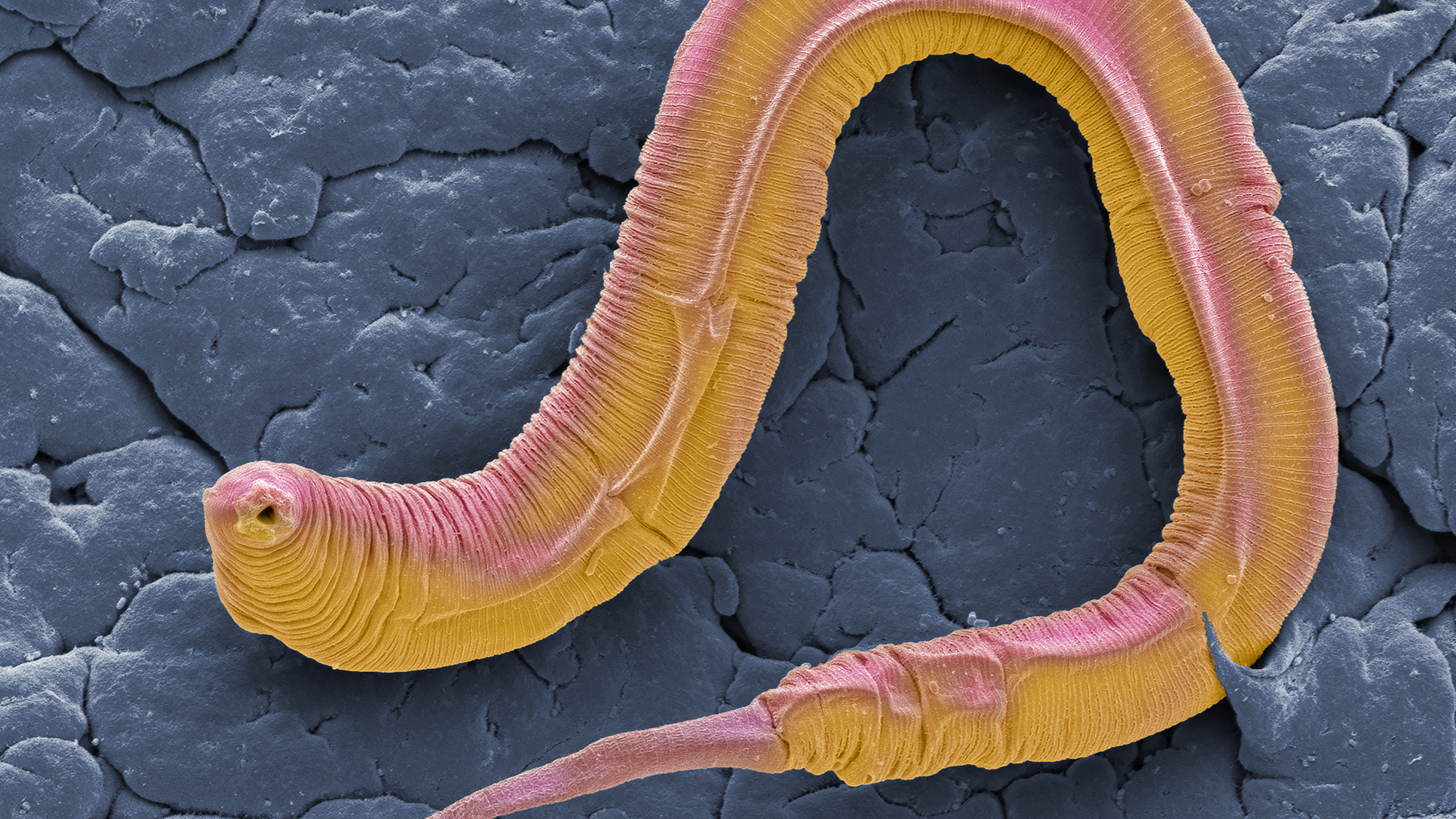
That lowly worm was the soil nematode Caenorhabditis elegans, introduced into modern biology by Sydney Brenner, a giant of the field known for his caustic wit. Born and initially educated in South Africa, he spent much of his productive life in Cambridge, England, before he established labs all over the world from California to Singapore, leading some of us to remark that the sun never set on the Brenner Empire. He first became famous for having discovered mRNA. More generally, he worked closely with Francis Crick on the nature of the genetic code and how it was read to make proteins. Once he and Crick decided that they’d solved the fundamental problem of using genetic information to make proteins, Brenner turned his attention to investigating how a complex animal develops from a single cell, and how the brain and its nervous system work.
Brenner identified C. elegans as an ideal organism to study because it could be grown easily, had a relatively short generation time, and was transparent, so you could see the cells that made up the worm. He trained a number of scientists at the MRC Laboratory of Molecular Biology in Cambridge and spawned an entire worldwide community of researchers studying C. elegans for everything from development to behavior. Among his colleagues was biologist John Sulston, whom you met in chapter 5. One of Sulston’s more remarkable projects was to painstakingly trace the lineage of each of the roughly nine hundred cells in the mature worm all the way from the single original cell, which led to an unexpected discovery: certain cells are programmed to die at precise stages of development. Scientists went on to identify the genes that sent these cells to commit suicide at just the right time in order for the organism to develop.
For an animal with only nine hundred cells, these worms are incredibly complex. They have some of the same organs as larger animals but in simpler form: a mouth, an intestine, muscles, and a brain and nervous system. They don’t have a circulatory or respiratory system. Though tiny—only about a millimeter long—nematodes can easily be seen wriggling around under a microscope. Being hermaphrodites, they produce both sperm and egg, but C. elegans can also reproduce asexually under some conditions. They are normally social, but scientists have found mutations that make them antisocial. Worms feed on bacteria, and just like bacteria, they are cultivated in petri dishes in the lab. They can be frozen away indefinitely in small vials in liquid nitrogen and simply thawed and revived when needed.
Worms typically live for a couple of weeks. However, when faced with starvation, they can go into a dormant state called dauer (related to the German word for endurance), in which they can survive for up to two months before reemerging when nutrients are plentiful again. Relative to humans’ life span, this would be the equivalent of three hundred years. Somehow these worms have managed to suspend the normal process of aging. There is a caveat, though: only juvenile worms can enter the dauer state. Once animals go through puberty and become adults, they no longer have this option.
David Hirsh became interested in C. elegans while he was a research fellow under Brenner at Cambridge, then continued working with the worms upon joining the faculty at the University of Colorado. There he took on a postdoc named Michael Klass, who wanted to focus on aging.
This was at a time when aging was simply thought to be a normal and inevitable process of wear and tear, and mainstream biologists viewed aging research with some disdain. However, things were beginning to change, partly because the US government was concerned about an aging population. As Hirsh recalled, the National Institutes of Health had just established the National Institute on Aging, and at least some of his and Klass’s motivation for working in the area was that they knew they stood a good chance of receiving federal funding.

Hirsh and Klass first showed that, by many criteria, worms age little if at all in the dauer state. Next, Klass wanted to see if he could isolate mutants of worms that would live longer but not necessarily go into dormancy. This would help him identify genes that affected life span. To rapidly produce mutants that he could screen for longevity, he treated the nematodes with mutagenic chemicals. He ended up with thousands of plates of worms, which he continued studying after starting his own lab in Texas. In 1983 Klass published a paper with a few long- lived mutant nematodes, but eventually he shut down his lab and joined Abbott Laboratories near Chicago. Before doing so, however, he sent a frozen batch of his mutant worms to a former colleague from Colorado, Tom Johnson, who by then was at the University of California, Irvine.
By inbreeding some of the mutant worms, Johnson found that their mean life span varied from ten to thirty-one days, from which he deduced that, at least in worms, life span involved a substantial genetic component. It still wasn’t clear how many genes affected life span, but in 1988 Johnson, working with an enthusiastic undergraduate student named David Friedman, came to a striking conclusion that ran completely counter to the conventional wisdom that many genes, each making small contributions, influenced longevity. Instead, it turned out that a mutation in a single gene, which the two called age-1, conferred a longer life span. Johnson went on to show that worms with the age-1 mutation had lower mortality at all ages, while their maximum life span more than doubled that of normal worms. Maximum life span, defined as the life span of the top 10 percent of the population, is considered a better measure of aging effects because mean life span can be affected by all sorts of other factors that don’t necessarily have to do with aging, such as environmental hazards and resistance to diseases.
