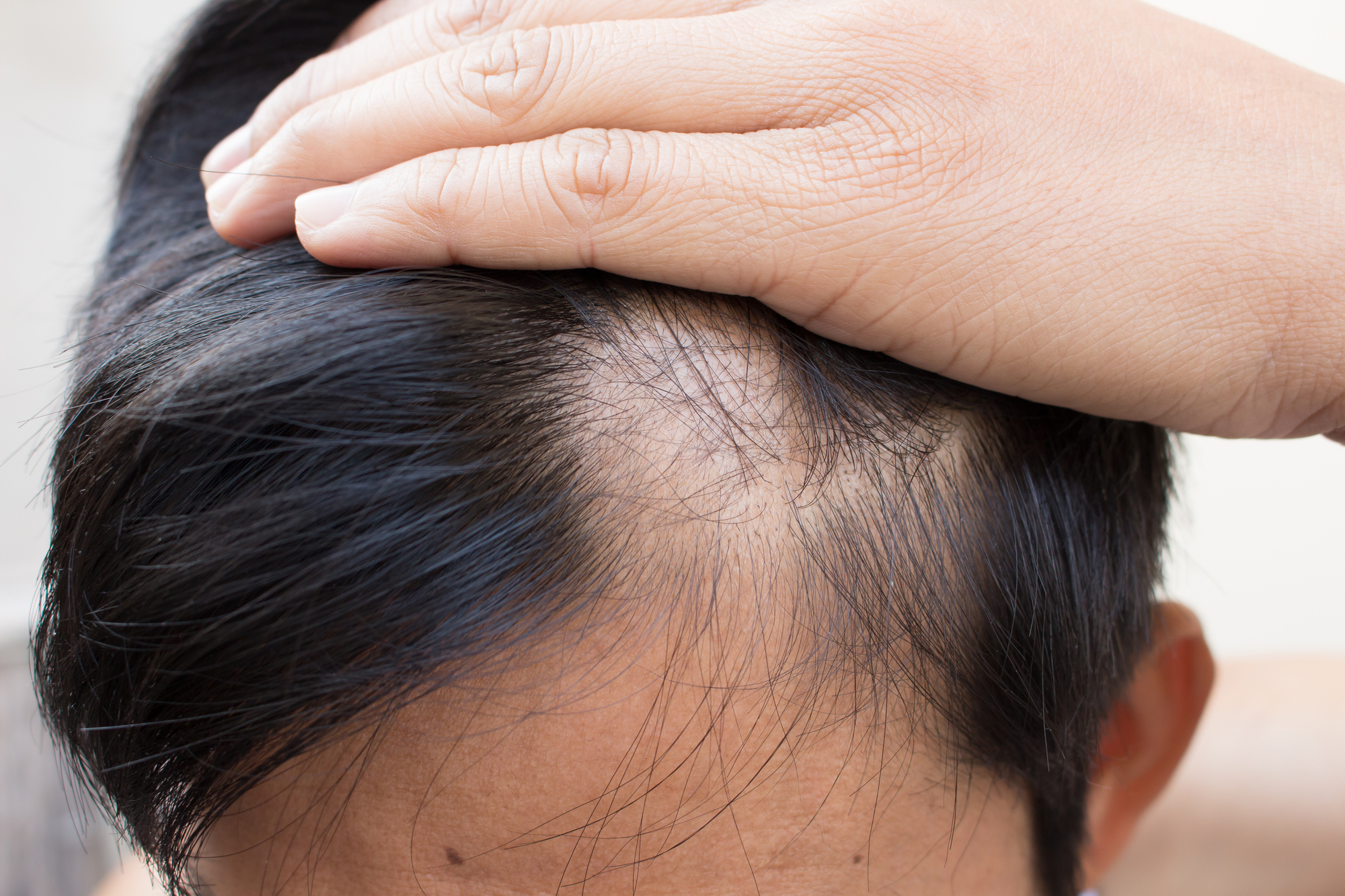

The Food and Drug Administration approved the first-ever systemic treatment for adults with severe alopecia areata on Monday. The drug, a JAK inhibitor that suppresses abnormal immune responses, may help over 300,000 people currently living with the skin disorder and could also prevent the condition in nearly 6.8 million Americans who are predicted to develop alopecia in the future.
Alopecia areata is an autoimmune disorder caused when the immune system attacks hair follicles, mistaking them as foreign invaders. The hair loss may begin as small circular bald patches around the head, but it can expand to larger overlapping patches.
Unexpected flare ups from extreme stress can worsen hair loss. The condition may affect a person’s self-image and can be a target of harassment, as when presenter Chris Rock bullied actor Jada Pinkett Smith for having alopecia at the Oscars in March.
Alopecia can be difficult to treat, and there is no cure. One therapy is corticosteroid injections every four to six weeks, which suppress the immune system to stop it from attacking hair follicles. However, the treatment is for milder cases—less than 50 percent of hair loss on the scalp or other body areas—and does not prevent new hair loss.
[Related: Scientists are trying to treat autoimmune disease with intestinal worms]
For more severe cases, a doctor may prescribe oral corticosteroids or topical immunotherapy. However, these medications carry a high risk of long-term side effects and aren’t approved by the FDA.
The newly approved drug, Olumiant (baricitinib), is part of a class of drugs called JAK inhibitors. In two Phase III clinical trials, 32 percent of people with severe alopecia who took 4 milligrams of Olumiant tablets had 80 percent of their hair grow back after 9 months. After a year, almost half of the patients grew their hair back. JAK inhibitors are different from prior alopecia treatments because they target inflammation all over the body instead of just the scalp. And Olumiant isn’t an injection–instead, patients take tablets by mouth.
“It isn’t practical to give injections in large areas without hair or in several locations on the body,” Suzanne Friedler, a dermatologist with Advanced Dermatology PC, told Healthline, referring to extensive forms of the disorder known as alopecia totalis or universalis. “These patients would benefit from oral medications. People with needle phobias are another group that would benefit.”
This style of treatment has another benefit, too: regrowing hair beyond the scalp. Alopecia also causes hair loss in the nose and ears, affecting a person’s hearing and allergies. A lack of eyelashes can promote eye irritation from dust freely entering the eye.
“It’s a beautiful thing to see the amazing impact,” Maryanne Makredes Senna, the director of the Hair Loss Center of Excellence at Beth Israel Lahey Health who uses JAK inhibitors in her practice, told the The New York Times. “They come in with no hair, totally withdrawing from life. Their eyes are cast down. They come back and say, ‘I have my life back. I have my self back.’”
The clinical trials that led to the FDA’s approval of Olumiant found it was safe and effective for people with severe alopecia. But like all drugs, it does carry some side effects, including abdominal pain, upper respiratory tract infections, and weight gain.
