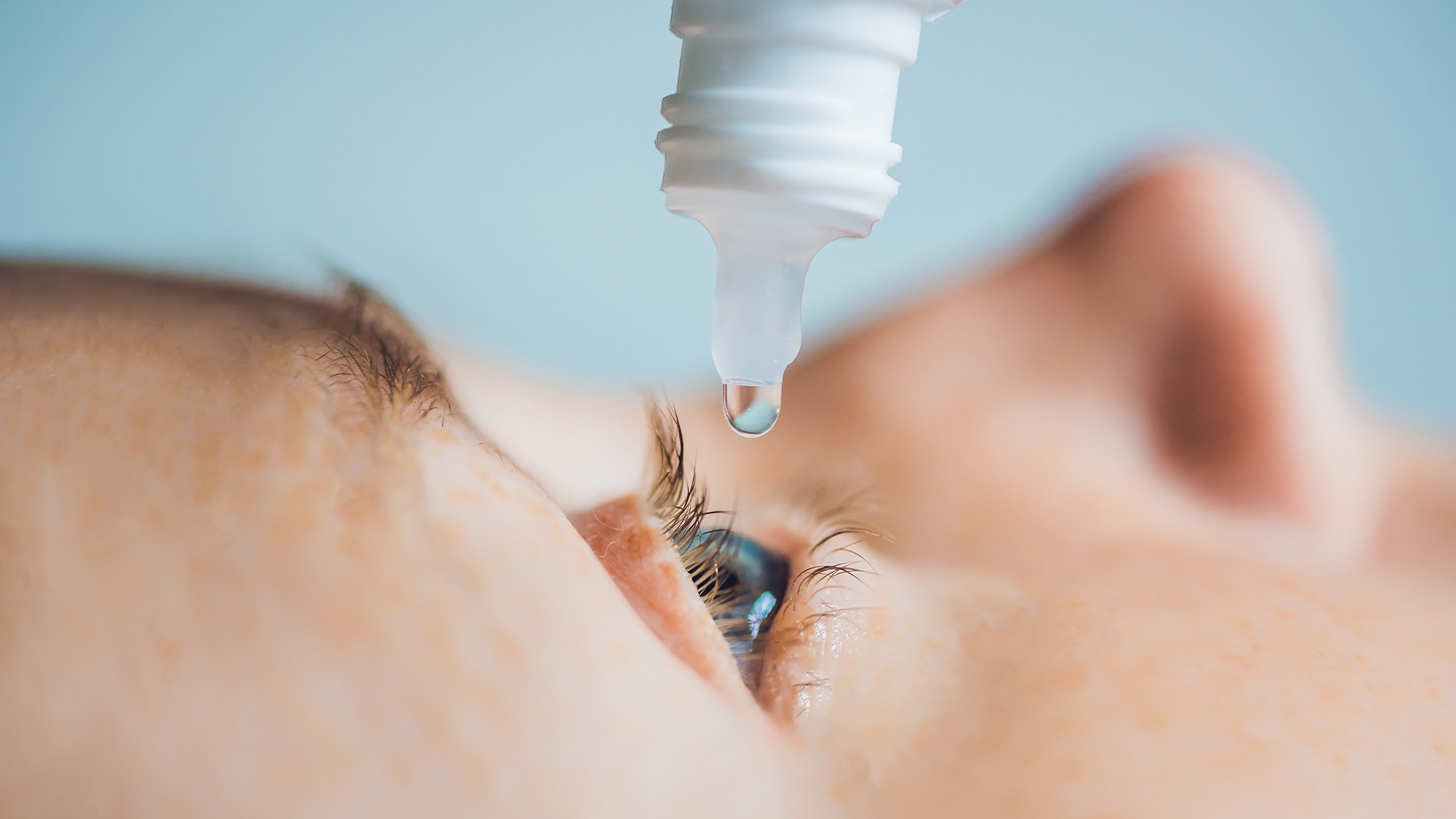

After several major recalls of eye drops and scathing inspection reports, the United States Food and Drug Administration (FDA) issued new guidance this week on the use of homeopathic eye drops. The agency warned that all drops labeled homeopathic should not be sold.
[Related: So, what’s the deal with homeopathy?]
Homeopathy can trace its root back to the Ancient Greeks, but was popularized by physician Christian Friedrich Samuel Hahnemann during the late 18th Century. Many of its methods are no better than a placebo. Homeopathy relies on two false principles. The false “law of similars” believes that something that causes a specific symptom in a healthy individual can also treat conditions and diseases with the same set of characteristics. The “law of infinitesimals” says that diluting a substance will make it more potent. Homeopathic products could take toxic substances and almost completely dilute them into more of an essence of the substance. There is no evidence to support the use of homeopathy. It can be very dangerous, as it keeps patients from seeking real medicine.
In November, Amazon stopped selling seven brands of eyedrops. The FDA sent a warning letter to Amazon CEO Andrew Jassy to stop selling “unapproved new drugs” at the major e-retailer. The products in question claimed to help pink eye, dry eyes, and eyestrain and the FDA says that these products violate federal regulations.
“These products are especially concerning from a public health perspective,” the FDA wrote in the letter. “Ophthalmic drug products, which are intended for administration into the eyes, in general pose a greater risk of harm to users because the route of administration for these products bypasses some of the body’s natural defenses.”
Eyes are at a greater risk of harm since they are one of the body’s immune-privileged sites. Immune responses are restrained in the eyes to prevent inflammation that can damage our vision.
Under the 1938 Food, Drug, and Cosmetic Act, homeopathic remedies are generally considered exempt from FDA safety and efficacy reviews if the active ingredient in the product is included in a list of substances approved by homeopaths. However, all seven of these unapproved products fall into the FD&C Act “because they are intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease, and/or intended to affect the structure or any function of the body.”
In October, the FDA also warned consumers about 26 over-the-counter eye drops from major brands sold at retailers like CVS and Target due to infection risk due to unsanitary conditions found at the manufacturers. Even though these drops are not all homeopathic, consumers should not buy any of the products and should immediately stop using them if they’ve already purchased them. The complete list can be found here.
The advisory posted by the FDA in October states that while no adverse effects had been reported for these products, the agency “found insanitary conditions in the manufacturing facility and positive bacterial test results from environmental sampling of critical drug production areas in the facility.”
[Related: Can our eyes ever fix themselves?]
The FDA is asking Congress for some new regulatory powers, including the ability to mandate drug recalls and require that the eyedrop manufacturers undergo and pass specific inspections before the products can be shipped to the US.
To find safe eye drops amid all of these recalls, consumers should verify where the drops were manufactured, double-check expiration dates, and look up specific products using an eye drop safety tool from Dry Eye Foundation.