New Metallic Glass Beats Steel as the Toughest, Strongest Material Yet
Materials scientists in California have made a special metallic glass with a strength and toughness greater than any known material,...
Materials scientists in California have made a special metallic glass with a strength and toughness greater than any known material, using a recipe that could yield a new method for materials fabrication.
The glass, a microalloy made of palladium, has a chemical structure that counteracts the inherent brittleness of glass but maintains its strength. It’s not very dense and it is more lightweight than steel, with comparable heft to an aluminum or titanium alloy.
“It has probably the best combination of strength and toughness that has ever been achieved,” said Robert O. Ritchie, a materials scientist at Lawrence Berkeley National Laboratory who is one of the authors of a paper describing the new glass. “It’s not the strongest material ever made, but it’s certainly one of the best with a combination of strength and toughness.”
In other words, some tougher materials exist, but they are less strong; there are stronger materials, but they’re not as tough. To grasp this, you have to define the the difference between strength and toughness. Strength refers to how much force a material can take before it deforms. Toughness explains the energy required to fracture or break something; it describes an object’s ability to absorb energy. Most of the time, these qualities are mutually exclusive. “The holy grail is to get both those properties at the same time,” Ritchie said.
Think of a ceramic mug — it’s pretty strong, maintaining its shape while handling hot and cold temperatures with ease. But it’s not very tough — there’s no give, no bendy quality to stop it from shattering when it falls to the floor. On the other hand, a rubber band is tough, stretching and contorting to wrap itself around your newspaper, your carton of eggs and a myriad other objects. But it’s weak, and it doesn’t take much energy for it to deform and break, snapping back on you with a painful recoil.
Souped-up glass is nothing new — Corning’s Gorilla Glass, which coats cell phones, laptops and TVs, is chemically strengthened with compressed ions, which helps prevent cracks and chips. Pyrex, used in telescope mirrors and baking dishes since 1915, is heat-strengthened to resist breakage. But neither has the toughness you’d want for making things like airplanes or bridges.
Ideal structural materials are both strong and tough; steel is a good example. The new glass has a far better combination of strength and toughness than any steel.
“When you build a structural material, you want it to be as strong as possible, but the limiting property is that it must be resistant to fracture, i.e., as tough as possible,” Ritchie said. “For instance, the Golden Gate Bridge is made of a relatively low-strength steel, because you’d like it to bend first rather than break catastrophically without warning.”
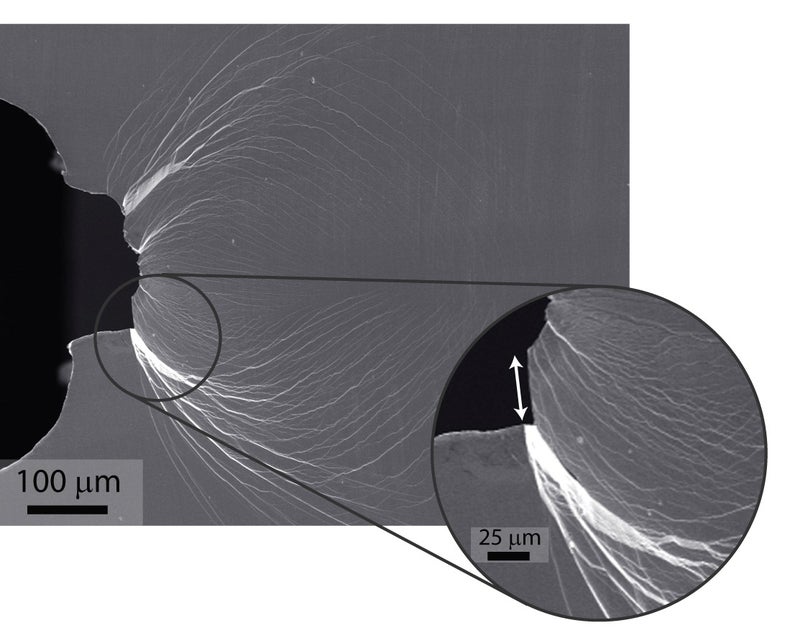
Glassy materials are usually very brittle — they break after the formation of shear bands, which are narrow zones of strain that ultimately become cracks. Once the bands form, it’s pretty much impossible to stop cracks from forming. But palladium’s properties change this dynamic, Ritchie explained. Instead of a single shear band propagating throughout the glass, a proliferation of shear bands form and curl back on themselves, taking longer to turn into cracks. The bands allow the material to bend before it breaks — not a property you’d expect from glass.
“It is very easy to form these shear bands, but it is difficult for them to become cracks. The net result is, you get a lot of shear bands forming, and this causes plasticity — you can bend it very readily,” Ritchie said.
Researchers at the California Institute of Technology, led by Marios D. Demetriou, have been working on metallic glass for several years, using various formulations to toughen it or prevent it from breaking. A previous iteration involved introducing a crystalline phase that stopped the shear bands in their tracks, for instance. The new glass has no crystals at all, just microalloys of palladium with phosphorous, silicon, germanium and silver.
“Each element wants to effectively crystallize in its own form, but if there are five, the material gets confused — it doesn’t know which way to crystallize, so the crystallization process is slowed down,” Ritchie said. “It’s 100 percent glass; there’s nothing to stop the cracks, and we think this is an important development.”
The Caltech researchers want to try it with other metal recipes next.
The glass is expensive and difficult to make because of the amount of metals involved and the process required to cool them. So you won’t start seeing palladium-glass airplanes and bridges anytime soon — but the material, and its fabrication process, holds promise for the future of those structures.
“For a bridge, a ship, a spacecraft, for engine material, you would like to combine strength and toughness. This does provide a means of doing that in quite frankly the most unlikely of all materials, a glass,” Ritchie said.
The new glass is described in this week’s issue of Nature Materials.