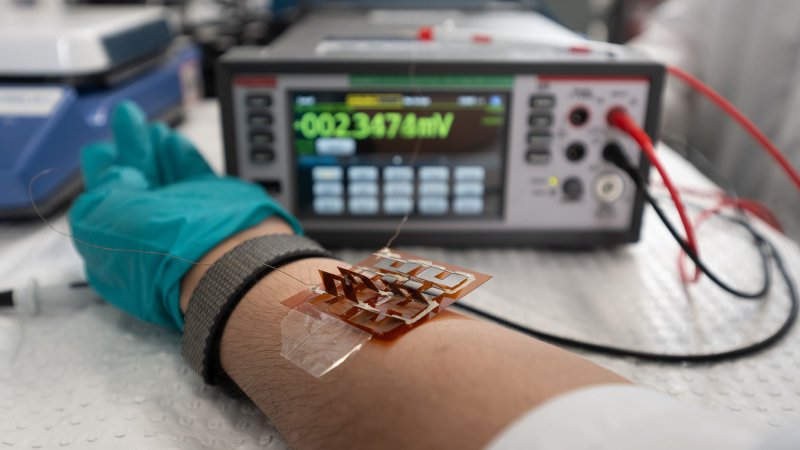
Technology
From our phones and smart assistants to transportation, the military, and cybersecurity—this is the latest on the technology shaping life on Earth and beyond.
Explore Technology
Latest in Technology
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
61
62
63
64
65
66
67
68
69
70
71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
87
88
89
90
91
92
93
94
95
96
97
98
99
100
101
102
103
104
105
106
107
108
109
110
111
112
113
114
115
116
117
Get the Popular Science newsletter
Breakthroughs, discoveries, and DIY tips sent every weekday.
By signing up you agree to our Terms of Service and Privacy Policy.