In 2011 You’ll Never Have to Clean Your House Again
Nanotechnology could soon allow you to sanitize your bathroom with a flip of a light switch
Launch the slideshow to see how titanium oxide reacts with light to zap dirt at the molecular level
Not so long ago, chemical engineers discovered how to use titanium dioxide to keep buildings free of discoloring pollution. Landmarks such as the virgin-white Dives in Misericordia Church in Rome and the Marunouchi Building in Tokyo were among the first to be coated with the semiconductor, which breaks down organic molecules-including those in grime and pollution-when exposed to light and water and then releases them into the air. Soon after, TiO2-based self-cleaning products, like SunClean windows from PPG Industries, hit the home market.
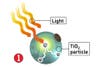
But to bring the technology inside the home, where it could eliminate the need for hours of tedious housework every week, researchers must overcome a major limitation: The technology currently responds only to ultraviolet light from the sun. Enter materials engineer Michael Cortie and his colleagues at the Institute for Nanoscale Technology in Sydney, Australia, who are working to perfect a coating that can respond to the visible spectrum-that is, the lightbulb hanging from your bathroom ceiling. So long, toilet brush.
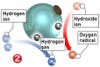
Two chemical qualities make TiO2 an all-purpose cleaner. First, the chemical is light-sensitive. When it is struck by photons, it reacts with air and water vapor to accelerate the breakdown of organic materials. It´s a bit like an artificial photosynthesis, but whereas plants use sunlight to break down carbon dioxide and turn it into oxygen, TiO2 uses light to turn scourges like grease and bacteria into carbon dioxide, hydrogen and other by-products that escape into the air. Second, TiO2 is hydro-philic, or water-loving. Instead of repelling water-as tiles and glass do when they encourage water to bead-materials coated with TiO2 attract water, causing it to â€sheet†across the surface, taking by-products and oversize particles with it. The result: Guck rarely gets a chance to build up, and it washes away easily when it does.
What´s needed to take the sun out of the equation? Cortie says TiO2´s atomic structure must be changed so that it´s compatible with the energy spectrum of visible light-no easy task. Plus, that alteration must be made without disrupting its chemical inertness; otherwise, it might not stay put on whatever it´s meant to coat.
Cortie is undeterred. And he´s convinced that TiO2 has a home market. â€Just look at the range of antibacterial sprays and wipes out there,†he says. â€People are demons for cleanliness. If it´s a product that doesn´t need to be sprayed-that´s just always there-even better.â€

by Kevin Hand
by Kevin Hand
by Kevin Hand
by Kevin Hand