NIH Is Testing A Potential Zika Vaccine In People
It contains genes that code for virus proteins
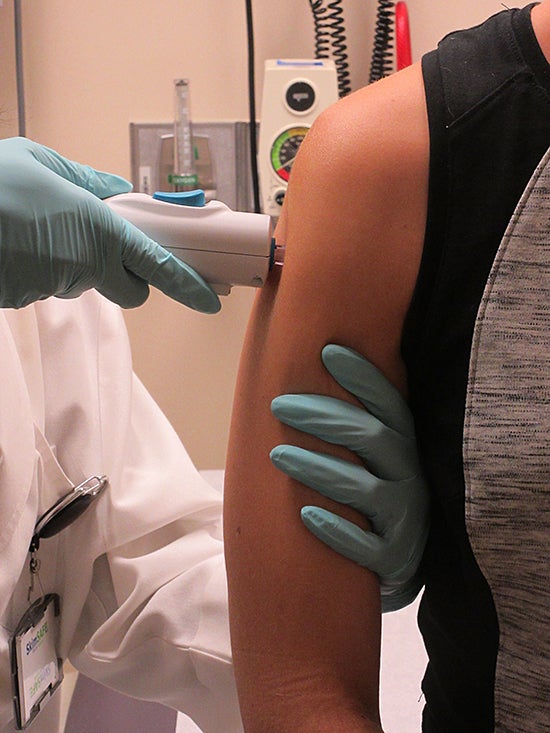
The National Institutes of Health announced today the start of a clinical trial to test out a vaccine to prevent the Zika virus in humans.
The new vaccine delivers genes, housed atop circular pieces of DNA called plasmids, that code for Zika virus proteins. The proteins will assemble into particles that resemble the virus (but can’t cause infection), inducing the body’s immune system to respond.
There are currently no vaccines or treatments for Zika, which causes mild or no symptoms in most people but can result in severe birth defects. Inovio Pharmaceuticals is also testing a potential vaccine this summer.
The NIH study will include at least 80 healthy volunteers, and focus on whether the vaccine is safe and able to trigger an immune response. If so (NIH expects to have results by the end of the year), the agency will begin a larger trial in countries where Zika is endemic.
