FDA Orders Personal Genomics Company 23andMe To Stop Marketing DNA Test
"FDA is concerned about the public health consequences of inaccurate results."
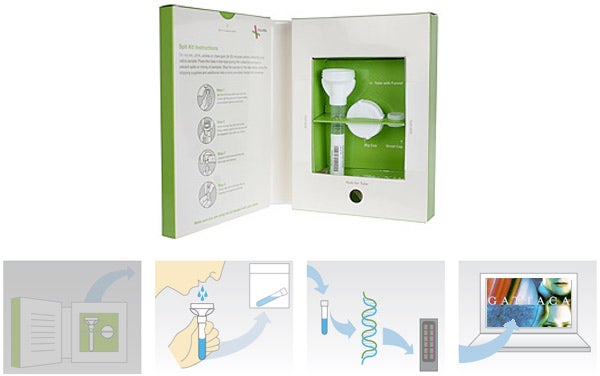
Since 2007, 23andMe has been selling a personal genomics test: using a kit, you submit your saliva for genetic sequencing, and receive a dataset all about your genealogy, disease risk factors, and so forth. Today, the Food and Drug Administration published a letter giving the company two weeks to discontinue marketing the kit, which the agency classifies as a medical diagnostic device in need of approval.
According to the letter, the FDA has been seeking information needed to approve the test for a while, “including more than 14 face-to-face and teleconference meetings, hundreds of email exchanges, and dozens of written communications”:
23andMe has not yet responded publicly.