How scientists are preparing for a world without antibiotics
5 strategies for beating antibiotic resistance
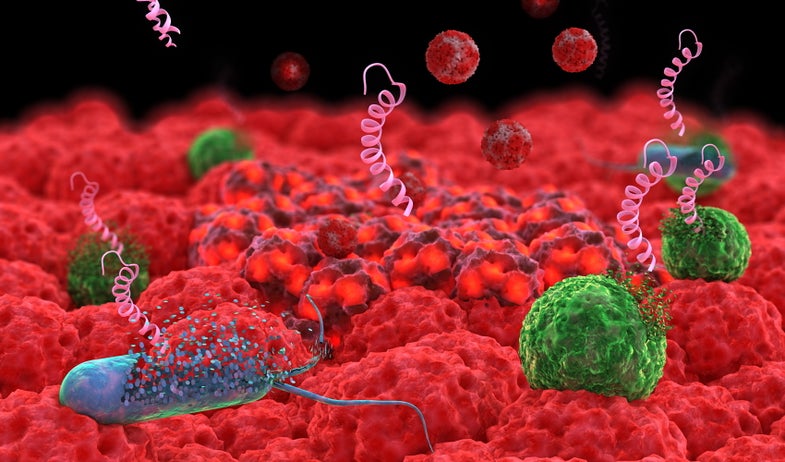
The drugs that have protected us from virulent bacteria for more than seventy years are slowly losing their edge, and we need new weapons. Disease-causing bacteria are becoming impervious to antibiotics that once wiped them out, including some drugs once considered last-resort.
Antibiotic-resistant bacteria infect at least 2 million people every year in the United States, killing 23,000. Some researchers estimate that if left unchecked, superbugs will kill 10 million people every year and cost the global economy $100 trillion by the year 2050.
“A lot of things that we take for granted right now, like say a C-section or a hip replacement or organ transplant…without having the antibiotics, these kinds of things will become really difficult,” says François Franceschi, a program officer for therapeutic development in the Bacteriology and Mycology Branch of the National Institute of Allergy and Infectious Diseases.
Those with weakened immune systems would be particularly vulnerable, but anyone could be at risk in a post-antibiotic world. “People are talking about potential for a post-antibiotic era where the antibiotics that we have available these days don’t work against simple infections like a small wound or a cut,” says César de la Fuente, a bioengineer at MIT.
To fight resistant bacteria, we are turning to new allies such as viruses that only attack bacteria, nanoparticles, and tiny proteins based on those produced by the immune systems of different organisms. Each tool comes with advantages and drawbacks, so researchers are exploring many approaches.
“A lot of people in the field have been now trying to look for alternative strategies that add to our arsenal,” says Timothy Lu, also of MIT. It’s “not that any one of them on their own is going to be the one silver bullet that’s going to cure bacteria for the rest of our lives, but more to be able to come at the problem from a variety of different ways.”
Here are some of the ways we will send our new allies into the fray in the war on superbugs.
Disarm the invaders
Bacteria don’t always need to be killed in order to be neutralized. Some treatments attack germs indirectly by targeting the weapons that make them virulent. “The bacteria will still be there but the consequences of the infection will not be severe, and then that will give the immune system…a chance to combat that infection,” Franceschi says.
If your drug doesn’t actually kill bacteria, they have less incentive to evolve resistance against it. “The development of resistance is going to take a lot longer because the bacteria isn’t actively fighting that,” Francsechi says.
Many bacteria secrete toxins that damage the cells of their host. One common type are called pore-forming toxins, which punch holes in cells. They’re produced by MRSA, Escherichia coli, Listeria, the bacteria that cause anthrax, and the venom of snakes, scorpions and sea anemones.
Liangfang Zhang has come up with a way to nullify these toxins. “You take away the weapon [and] they can become much weaker,” says Zhang, a nanoengineer at the University of California, San Diego. He coats nanoparticles with an irresistible target—membranes plucked from red blood cells. The red blood cell shell acts as a decoy, drawing in toxins that would otherwise attack healthy cells. “They serve as a sponge to suck up all these toxins,” Zhang says.
In their first exploration, the nanosponges soaked up the toxins without harming mice. This year, Zhang’s work with nanoparticles as decoys was one of 24 projects awarded funding by the National Institutes of Health. He hopes to begin human clinical trials in the next year to two.
Nanoparticles, which are often made from plastics or metals such as silver, can also impair bacteria by disrupting their protective cell membranes or causing DNA damage. Nanoparticles are easy to work with because they essentially build themselves. “You control the temperature, you control the solvent and so on, these molecules will automatically assemble into the nanoparticle,” Zhang says.
Nanoparticles may be more expensive than traditional antibiotics. And directing them to the right spot in a body is also a challenge. Another concern is making sure that the nanoparticles are made from materials that won’t trigger an immediate immune response, and will break down over time so they don’t accumulate in the body.
“There are still ongoing questions about the long term safety of some of these things,” Lu says. “That being said I think using nanoparticles really can have an antimicrobial effect that’s very potent.”
Special delivery
Alternative therapies can also be used to make existing antibiotics more effective. Scientists are investigating how to use nanoparticles to deliver cancer drugs and antibiotics.
Antibiotics spread throughout the body and are toxic in high doses. With nanoparticles, you can deploy concentrated hits of drugs. Thousands of drug molecules can be stuffed inside a single nanoparticle.
“They can easily just stick to the membrane and constantly release the drug right onto the bacteria,” Zhang says. This means a larger, more effective cargo can be used without boosting the total drug dose.
“This can overwhelm the bacteria’s resistance mechanisms, because they haven’t developed resistance mechanisms against this drug storm,” Zhang says.
A problem with nanoparticles, and many other tools, is that the immune system sees them as a threat. “The size is very much like viruses,” Zhang says. “Our body actually is trained to clear these nanoparticles or viruses if you don’t protect them.”
Zhang and his colleagues have camouflaged nanoparticles in jackets made from the membranes of platelets—cell fragments that help blood clot. “From outside it looks just like a mini cell,” Zhang says.
Certain bacteria are attracted to platelets, which they hijack to mask themselves from the immune system. Platelet-coated nanoparticles turn the table on these bacteria, luring the interlopers in only to blast them with drugs.
“All the nanoparticles will specifically go to the bacteria and release the drug,” says Zhang. He has used the platelet-coated particles to treat mice infected with a strain of MRSA, which is resistant to many antibiotics.
Direct attack
Sometimes, however, no subterfuge is needed. Many of the alternatives to traditional antibiotics can kill bacteria outright. One strategy is designing manmade versions of antimicrobial peptides (AMPs), which are a part of the innate immune response in microbes, plants and animals (such as Tasmanian devils). These compounds attack a pathogen’s membrane and may also wreak havoc inside the cell.
In a recent project, de la Fuente collaborated with Lu and others to select a non-toxic AMP discovered in simple marine animals called tunicates. The team added a few amino acids to this template, improving its ability to treat mice infected with antibiotic-resistant E. coli and MRSA. The souped-up AMP also galvanized the rodents’ immune systems by calming inflammation and calling for backup in the form of white blood cells.
Antimicrobial peptides can vanquish a wide range of pathogens, and bacteria have difficulty developing resistance to them. “In comparison with conventional antibiotics, these peptides are more effective in many cases,” de la Fuente says.

AMPs are made from relatively short strings of amino acids, the building blocks of proteins. This makes them straightforward (albeit time-consuming and expensive) to build. “We have yet to bring down the cost,” de la Fuente says. Researchers are exploring ways to build AMPs more cheaply by programming microbes to make them instead of relying on a machine.
However, there are concerns that AMPs might turn on host cells. And as with many alternatives to antibiotics, sending them to the right location at a high enough concentration to be effective is also a challenge. “What’s more feasible in the short term is probably topical application,” de la Fuente says. “We would formulate these peptides into, say, a cream that you could apply [if] you have a skin infection or an open wound.” They could also be used to coat tables, computers, surgical instruments, or catheters to prevent them from being colonized by germs.
Re-sensitization
Another way to weaken bacteria is to knock out the resistance they have cultivated against antibiotics. Viruses that are specialized to prey on bacteria, called bacteriophages, can be tapped for these missions.
Phages are extremely effective bacteria-killers, but researchers can use genetic engineering to give them new skills to bring to battle, and to restore bacteria’s sensitivity to traditional drugs.
Reprogrammed phages may lock onto bacteria carrying genes that confer antibiotic resistance, erasing this ability or killing the bacteria. With the resistant germs knocked out or defused, the remaining population is vulnerable to antibiotics.
Another way bacteria resist antibiotics is by secreting compounds that create a barrier called a biofilm that drugs cannot penetrate. Phages can be engineered to chew up the biofilm (Lu and de la Fuente’s redesigned antimicrobial peptide also shows promise for biofilm busting).
In the wild, phages can slay bacteria directly. “Some phages will introduce their DNA into the bacteria and in order to liberate themselves… just chew up the cell wall, for example, and sort of explode the cell,” Lu says. Others act as parasitic hitchhikers.
Modified phages can also wipe out bacteria. “What we have been doing is trying to engineer phages that can go into a bacteria and kill them in a very targeted way,” Lu says. “You can introduce new functions into the phages to make them more powerful antimicrobial agents.”
Phages were actually discovered about 100 years ago. They were eclipsed by antibiotics in the United States, although some areas of Eastern Europe have continued to use them. Currently, there are no FDA-approved phages, although clinical trials are underway. Phages seem to be about as effective as antibiotics in treating humans, although there is not yet clinical data to confirm this.
One advantage of these viruses is that they can make more of themselves (as viruses are wont to do once placed inside a host). “You can put a small amount in and kill a lot of bacteria,” Lu says. And because they need living bacterial cells to reproduce, they’re unlikely to stick around once their hosts have been wiped out.
However, like other alternatives, phages may trigger the immune system. “If you inject any virus or any foreign peptide into a person, there’s always a chance there’s going to be a reaction,” Lu says. Another worry is that some phages may pick up genes related to antibiotic resistance and transfer them to other bacteria.
But they’re unlikely to harm human tissue. “Phages do not replicate in human cells, and I haven’t seen any reports where they’ve been shown to have any negative consequences,” Lu says. “There are tons of phages inside of us already, it’s not like these things are foreign to us.”
A personal touch
Some alternative therapies may be tailored to fight specific germs. Here, again, phages are ideal candidates. “They are basically the natural enemy of bacteria,” Lu says. Generally, “if you find a bacteria, you can find a phage against it.”
Traditional antibiotics often kill bacteria indiscriminately—including those in our body’s natural microbiome that play key roles in our health. This can leave an opening for opportunists like Clostridium difficile to colonize the body. “You don’t want to carpet bomb the gut and kill all the bacteria,” Franceschi says.
Viruses offer a more personalized approach. “You can try to spare the good bacteria…while still being able to kill the bad bacteria,” Lu says.
But this specificity is a double-edged sword. To cover enough of the different bacteria that might be infecting a patient, multiple viruses will have to be mixed into a cocktail. And, while phages aren’t particularly expensive to grow, cocktails laced with many viruses make manufacturing more complicated. “The traditional way of doing that has been to go into nature and just find all the different phages and mix them together,” Lu says. “It poses a lot of challenges in terms of practical development.”
Lu is working on an NIH-funded project to make cocktails full of phages built from the safe scaffold. By tweaking the region that dictates what a phage infects, you can target different bacteria without modifying the rest of the virus. “That allows you to take phages and point them in different directions,” Lu says. “That’s one of the last remaining hurdles for phage therapy to become widespread, the ability to make sort of a well defined cocktail and tune it so it will go after bacteria you care about.”
Even so, it’s difficult to craft a tailored medicine without knowing what is causing an infection. “If you go to a doctor, they’re…not going to feel confident giving you a narrow spectrum treatment if they don’t actually know what the bacteria is,” Lu says.
Doctors need speedier diagnostics so they can figure out which bacteria they are going after, and whether it is resistant to traditional antibiotics. Lu and his colleagues have engineered phages for quick, cheap diagnostics. When they infect their target bacteria, they light up by producing the same protein that fireflies use. Expose phages to sample from your patient, and “you can simply read out whether the sample is glowing or not, and then you know whether that bacteria was present in that sample,” Lu says.
Doctors can then use tailored therapies, whether they are made from phages or other tools. “You really need that option of diagnostics or none of the vision of having narrow spectrum antimicrobials is going to work,” Lu says.
A diverse armory
These aren’t the only weapons we are adding to our cache. Researchers are also exploring other options, like sending other bacteria to fight pathogens, continuing to hunt for new antibiotics (often inspired by compounds bacteria use to kill each other in the wild), and using antibodies, among other things.
“You probably can’t just rely on one technology, or one thing, to eradicate the entire problem,” Zhang says. Tackling superbugs from many angles, sometimes even combining new tactics with traditional therapies, will give doctors options to choose from.
It’s going to be a few years before these new tools are adopted for widespread use. And for awhile, these alternative antimicrobials will be directed to cases where antibiotics are no longer working. “Antibiotics, when they do work, are so cheap and so awesome that I think getting clinicians to move away from that wholesale would be really hard,” Lu says. “Long term, my hope…is really that it will replace a lot of the broad spectrum antimicrobials because…messing up our microbiome is bad for us in a lot of different ways, and I think the only solution for that is really to get to targeted therapy.”
Sending a diverse array of weapons against bacteria will slow down the development of resistance – the wide use of individual antibiotics has made it relatively simple for the bacteria they target to evolve defenses – but it won’t make the problem go away.
“Bacteria are essentially very plastic and vey well equipped to evolve really quickly,” Franceschi says. “Bacteria will keep evolving and you will always need something new.”
